Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection
- PMID: 33636554
- PMCID: PMC7891078
- DOI: 10.1016/j.jcv.2021.104765
Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection
Abstract
Background: Most SARS-CoV-2 infected patients develop IgG antibodies within 2-3 weeks after symptom onset. Antibody levels have been shown to gradually decrease in the first months after infection, but few data are available at six months or later.
Methods: A retrospective multi-center study was performed using 652 samples of 236 PCR-confirmed SARS-CoV-2 infected patients from 2 Belgian University hospitals. Patients were included if at least two samples were available (range 2-7 samples); including at least one sample collected 30 days or later after first positive PCR (range 0-240 days). Of those 236 patients, 19.1 % were classified as mild/asymptomatic (mild) and 80.9 % as moderate to critical (severe). IgG anti-nucleocapsid antibodies (anti-N) were measured using the Abbott Architect immunoassay.
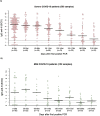
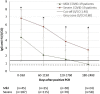
Results: 22.2 % of mild and 2.6 % of severe COVID-19 cases never seroconverted (p < 0.001). Of the mild patients who seroconverted 0-59 days after PCR; 18.8 %, 40.0 % and 61.1 % were seronegative in the windows 60-119 days, 120-179 days and 180-240 days after PCR, respectively. In severe patients, these numbers were 1.9 %, 10.8 % and 29.4 % respectively (p < 0.05 each). Antibody levels were significantly higher in severe patients compared to mild patients in each 60 day window (p < 0.001 each).
Conclusions: SARS-CoV-2 anti-N IgG antibody levels steadily decreased after 2 months up to 8 months post PCR. Of severe COVID-19 patients, 70.6 % remained positive up to eight months after infection. Antibody levels were significantly lower in mild SARS-CoV-2 infected patients and 61.1 % became seronegative within 6 months after the first positive PCR.
Keywords: Antibodies; COVID-19; Coronavirus; Immunoglobulin G; Longitudinal studies; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
-
- Van Elslande J., Houben E., Depypere M., Brackenier A., Desmet S., André E., Van Ranst M., Lagrou K., Vermeersch P. Diagnostic performance of 7 rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin. Microbiol. Infect. 2020;26:1082–1087. doi: 10.1016/J.CMI.2020.05.023. - DOI - PMC - PubMed
-
- Van Elslande J., Decru B., Jonckheere S., Van Wijngaerden E., Houben E., Vandecandelaere P., Indevuyst C., Depypere M., Desmet S., André E., Van Ranst M., Lagrou K., Vermeersch P. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by 4 automated immunoassays and 3 ELISAs. Clin. Microbiol. Infect. 2020;26(1557):e1–1557. doi: 10.1016/j.cmi.2020.07.038. e7. - DOI - PMC - PubMed
-
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O’Byrne A., Kouphou N., Galao R.P., Betancor G., Wilson H.D., Signell A.W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J.M., Lista M.J., Temperton N., Snell L.B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M.K.I., O’Connell L., O’Hara G., MacMahon E., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J.D., Neil S.J.D., Malim M.H., Doores K.J. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. - DOI - PMC - PubMed
-
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

