Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis
- PMID: 33637050
- PMCID: PMC7908737
- DOI: 10.1186/s12879-021-05850-0
Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis
Erratum in
-
Correction to: Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis.BMC Infect Dis. 2021 Apr 12;21(1):340. doi: 10.1186/s12879-021-06016-8. BMC Infect Dis. 2021. PMID: 33845777 Free PMC article. No abstract available.
Abstract
Background: The objective of this study was to assess the durability of response of dolutegravir (DTG) as an antiretroviral core agent by comparing its efficacy and safety with other recommended or commonly used core agents up to 96-weeks (W96).
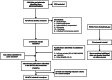
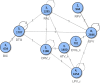
Methods: A previously published systematic review was updated to identify phase 3/4 randomised controlled trials (RCTs) of core agents in treatment-naïve HIV-1 patients. Efficacy [virologic suppression (VS), CD4+ cell change from baseline] and safety [adverse events [AEs], discontinuations, drug-related AEs [DRAEs]] were analysed at W96 using Bayesian network meta-analysis (NMA) adjusting for nucleoside/nucleotide reverse transcriptase inhibitors' (NRTIs') backbone. Subgroups of patients with VL > 100,000 copies/mL or CD4+ ≤ 200 cells/μL at baseline were analysed separately.
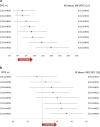
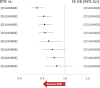
Results: The NMA included 20 studies reporting data at W96. A higher proportion of patients receiving DTG achieved VS compared to those on protease inhibitors [PI:Range:8.7%(CrI:3.1,16.0)-19.9%(10.8,30.5)], efavirenz [EFV:6.9%(1.3,10.8)] and cobicistat-boosted elvitegravir [EVG/c:8.2%(0.2,17.4)], and similar but numerically higher compared to rilpivirine [RPV:5.0%(- 2.8,12.5)], raltegravir [RAL:2.9%(- 1.6,7.7)] and bictegravir [BIC:2.7%(- 2.7,10.6)]. The probability that more patients on DTG would achieve VS at W96 compared to any other core agent was greater than 80%. A higher proportion of patients on DTG achieved VS compared to PI/rs [Range:33.1%(13.6,50.4)-45.3%(24.1,61.6)] and RAL [16.7%(3.3,31.2)] in patients with VL > 100,000 copies/mL at baseline, and similar VS was achieved in patients with CD4+ ≤ 200 cells/μL at baseline. DTG also achieved greater increase in CD4+ cells from baseline compared to EFV [32.6(10.7,54.7)], ritonavir-boosted darunavir [DRV/r:25.7(3.6,48.1)] and BIC [24.7(1.5,47.7)]. Patients receiving DTG had lower odds of discontinuing therapy by W96 compared to PI/rs, EFV, RAL and EVG/c. Patients on DTG had lower odds of experiencing an adverse event (AE) compared to patients on EFV [odds ratio:0.6(0.3,0.9)], ATV/r [0.4(0.3,0.6)] and LPV/r [0.3(0.2,0.5)]. For patients on DTG, the odds of experiencing a drug-related AE were lower than the odds for patients on EFV [0.3(0.2,0.4)], comparable to patients on RAL [1.1(0.8,1.4)] and higher than those on BIC [1.5(1.1,2.0)].
Conclusion: Un-boosted integrase inhibitors had better efficacy and similar safety compared to PI/rs at W96 in treatment-naïve patients with HIV-1, with DTG being among the most efficacious core agent, particularly in patients with baseline VL > 100,000 copies/mL or ≤ 200 CD4+ cells/μL, who can be difficult to treat.
Keywords: Antiretroviral therapy; Dolutegravir; HIV-1; Integrase strand inhibitors; Network meta-analysis; Non-nucleoside reverse transcriptase inhibitor; Protease inhibitor; Systematic review; Treatment-naïve.
Conflict of interest statement
KN, NJAH and SJS are employees of Pharmerit International and were contracted to conduct this study on behalf of ViiV Healthcare. YSP is an employee of ViiV Healthcare and holds stock in GlaxoSmithKline.
Figures
References
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC).Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV (updated December 2019). https://files.aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolesc.... Accessed 02 Apr 2020.
-
- European AIDS Clinical Society . Guidelines. Version 10.0. European AIDS Clinical Society: Brussels, Belgium; 2019.
-
- WHO 2018. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviraldrugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018. (WHO/CDS/HIV/18.51). Licence: CC BY-NC-SA 3.0 IGO
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

