Aerosol drug delivery to spontaneously-breathing preterm neonates: lessons learned
- PMID: 33637075
- PMCID: PMC7908012
- DOI: 10.1186/s12931-020-01585-9
Aerosol drug delivery to spontaneously-breathing preterm neonates: lessons learned
Abstract
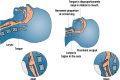
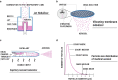
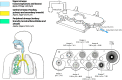
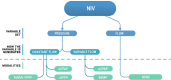
Delivery of medications to preterm neonates receiving non-invasive ventilation (NIV) represents one of the most challenging scenarios for aerosol medicine. This challenge is highlighted by the undersized anatomy and the complex (patho)physiological characteristics of the lungs in such infants. Key physiological restraints include low lung volumes, low compliance, and irregular respiratory rates, which significantly reduce lung deposition. Such factors are inherent to premature birth and thus can be regarded to as the intrinsic factors that affect lung deposition. However, there are a number of extrinsic factors that also impact lung deposition: such factors include the choice of aerosol generator and its configuration within the ventilation circuit, the drug formulation, the aerosol particle size distribution, the choice of NIV type, and the patient interface between the delivery system and the patient. Together, these extrinsic factors provide an opportunity to optimize the lung deposition of therapeutic aerosols and, ultimately, the efficacy of the therapy.In this review, we first provide a comprehensive characterization of both the intrinsic and extrinsic factors affecting lung deposition in premature infants, followed by a revision of the clinical attempts to deliver therapeutic aerosols to premature neonates during NIV, which are almost exclusively related to the non-invasive delivery of surfactant aerosols. In this review, we provide clues to the interpretation of existing experimental and clinical data on neonatal aerosol delivery and we also describe a frame of measurable variables and available tools, including in vitro and in vivo models, that should be considered when developing a drug for inhalation in this important but under-served patient population.
Keywords: Aerosol delivery; Nebulizer; Non-invasive ventilation; Premature infants; Pulmonary drug delivery; Respiratory distress syndrome; Surfactant.
Conflict of interest statement
FB, FS, IM, SB, and EP are employees of Chiesi Farmaceutici S.p.A. XM served as consultant for this study.
Figures

References
-
- Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015;169:1011–1017. doi: 10.1001/jamapediatrics.2015.2401. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

