Cryoneurolysis for the management of chronic pain in patients with knee osteoarthritis; a double-blinded randomized controlled sham trial
- PMID: 33637085
- PMCID: PMC7913284
- DOI: 10.1186/s12891-021-04102-1
Cryoneurolysis for the management of chronic pain in patients with knee osteoarthritis; a double-blinded randomized controlled sham trial
Abstract
Objective: Pain is the principal symptom in knee osteoarthritis (OA). Current non-operative treatment options have only moderate effects and often patients experience persistent pain or side-effects. Novel advances in the field of cryoneurolysis applies low temperatures to disrupt nerve signaling at the painful area, providing pain relief. The primary aim of this randomized controlled trial (RCT) is to investigate if cryoneurolysis is superior to sham at decreasing pain intensity 2 weeks after the intervention in patients with knee OA. Secondary aims are to explore effects on pain, quality of life and functional performance over 24 months.
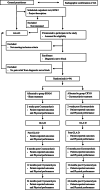
Methods: This two-arm, parallel-group RCT, approved by the Regional Ethics Committee, will randomly allocate patients (n = 94) to a cryoneurolysis intervention group + standardized education and exercise (CRYO) or a sham group + standardized education and exercise (SHAM) (1:1 ratio). Both groups will be assessed at baseline, 2 weeks post intervention, post education and exercise and at 6, 12 and 24 months after cryoneurolysis. The primary outcome is the NRS knee pain intensity score assessed 2 weeks post the intervention. Secondary outcome measures include functional performance (chair-stand test, 40 m walk, stair test and maximum voluntary contraction of the knee), patient reported outcomes (quality of life (EQ5D), Knee and osteoarthritis outcome scores (KOOS), among others), use of analgesics, and adverse events over 24 months.
Impact statement: Cryoneurolysis could potentially provide an effective, safe and non-pharmacological therapeutic option to treat pain in OA patients. The potential benefits include increased functional capacity and quality of life as a result of significant pain relief and improved benefits of physical exercise.
Trial registration: Clinicaltrials.gov, NCT03774121 , registered 3 March 2018, http://www.clinicaltrials.gov.
Keywords: Cryoneurolysis; Exercise; Knee pain; Osteoarthrities.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- O’Brien T, Breivik H. The impact of chronic pain—European patients’ perspective over 12 months. 2012. pp. 23–29. - PubMed
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical