Ketamine Rapidly Enhances Glutamate-Evoked Dendritic Spinogenesis in Medial Prefrontal Cortex Through Dopaminergic Mechanisms
- PMID: 33637303
- PMCID: PMC8740507
- DOI: 10.1016/j.biopsych.2020.12.022
Ketamine Rapidly Enhances Glutamate-Evoked Dendritic Spinogenesis in Medial Prefrontal Cortex Through Dopaminergic Mechanisms
Abstract
Background: Ketamine elicits rapid onset antidepressant effects in patients with clinical depression through mechanisms hypothesized to involve the genesis of neocortical dendritic spines and synapses. Yet, the observed changes in dendritic spine morphology usually emerge well after ketamine clearance, raising questions about the link between rapid behavioral effects of ketamine and plasticity.
Methods: Here, we used two-photon glutamate uncaging/imaging to focally induce spinogenesis in the medial prefrontal cortex, directly interrogating baseline and ketamine-associated plasticity of deep layer pyramidal neurons in C57BL/6 mice. We combined pharmacological, genetic, optogenetic, and chemogenetic manipulations to interrogate dopaminergic mechanisms underlying ketamine-induced rapid enhancement in evoked plasticity and associated behavioral changes.
Results: We found that ketamine rapidly enhances glutamate-evoked spinogenesis in the medial prefrontal cortex, with timing that matches the onset of its behavioral efficacy and precedes changes in dendritic spine density. Ketamine increases evoked cortical spinogenesis through dopamine Drd1 receptor (Drd1) activation that requires dopamine release, compensating blunted plasticity in a learned helplessness paradigm. The enhancement in evoked spinogenesis after Drd1 activation or ketamine treatment depends on postsynaptic protein kinase A activity. Furthermore, ketamine's behavioral effects are blocked by chemogenetic inhibition of dopamine release and mimicked by activating presynaptic dopaminergic terminals or postsynaptic Gαs-coupled cascades in the medial prefrontal cortex.
Conclusions: Our findings highlight dopaminergic mediation of rapid enhancement in activity-dependent dendritic spinogenesis and behavioral effects induced by ketamine.
Keywords: 2-Photon glutamate uncaging; Dendritic spine; Dopamine; Ketamine; Medial prefrontal cortex; Spinogenesis.
Copyright © 2021 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no biomedical financial interests or potential conflicts of interest. A part of this study, along with additional data, has been posted on bioRxiv:
Figures
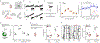
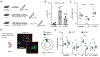
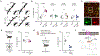

Comment in
-
Ketamine for a Boost of Neural Plasticity: How, but Also When?Biol Psychiatry. 2021 Jun 1;89(11):1030-1032. doi: 10.1016/j.biopsych.2021.03.014. Biol Psychiatry. 2021. PMID: 34016377 Free PMC article. No abstract available.
Similar articles
-
Attenuated dopamine signaling after aversive learning is restored by ketamine to rescue escape actions.Elife. 2021 Apr 27;10:e64041. doi: 10.7554/eLife.64041. Elife. 2021. PMID: 33904412 Free PMC article.
-
Estradiol-Mediated Spine Changes in the Dorsal Hippocampus and Medial Prefrontal Cortex of Ovariectomized Female Mice Depend on ERK and mTOR Activation in the Dorsal Hippocampus.J Neurosci. 2016 Feb 3;36(5):1483-9. doi: 10.1523/JNEUROSCI.3135-15.2016. J Neurosci. 2016. PMID: 26843632 Free PMC article.
-
Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation.Science. 2019 Apr 12;364(6436):eaat8078. doi: 10.1126/science.aat8078. Science. 2019. PMID: 30975859 Free PMC article.
-
Mechanisms of ketamine action as an antidepressant.Mol Psychiatry. 2018 Apr;23(4):801-811. doi: 10.1038/mp.2017.255. Epub 2018 Mar 13. Mol Psychiatry. 2018. PMID: 29532791 Free PMC article. Review.
-
Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies.Pharmacol Ther. 2019 Jul;199:58-90. doi: 10.1016/j.pharmthera.2019.02.017. Epub 2019 Mar 7. Pharmacol Ther. 2019. PMID: 30851296 Review.
Cited by
-
Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum.Nat Commun. 2021 Aug 11;12(1):4855. doi: 10.1038/s41467-021-25144-y. Nat Commun. 2021. PMID: 34381044 Free PMC article.
-
Synaptic Mechanisms Regulating Mood State Transitions in Depression.Annu Rev Neurosci. 2022 Jul 8;45:581-601. doi: 10.1146/annurev-neuro-110920-040422. Epub 2022 May 4. Annu Rev Neurosci. 2022. PMID: 35508195 Free PMC article. Review.
-
Shared and Distinct Brain Regions Targeted for Immediate Early Gene Expression by Ketamine and Psilocybin.ACS Chem Neurosci. 2023 Feb 1;14(3):468-480. doi: 10.1021/acschemneuro.2c00637. Epub 2023 Jan 11. ACS Chem Neurosci. 2023. PMID: 36630309 Free PMC article.
-
Cocaine and habit training cause dendritic spine rearrangement in the prelimbic cortex.iScience. 2023 Feb 20;26(4):106240. doi: 10.1016/j.isci.2023.106240. eCollection 2023 Apr 21. iScience. 2023. PMID: 37153443 Free PMC article.
-
Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo.Neuron. 2021 Aug 18;109(16):2535-2544.e4. doi: 10.1016/j.neuron.2021.06.008. Epub 2021 Jul 5. Neuron. 2021. PMID: 34228959 Free PMC article.
References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000): Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. - PubMed
-
- Kim J, Farchione T, Potter A, Chen Q, Temple R (2019): Esketamine for Treatment-Resistant Depression — First FDA-Approved Antidepressant in a New Class. N Engl J Med 381: 1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

