Late-stage stitching enabled by manganese-catalyzed C─H activation: Peptide ligation and access to cyclopeptides
- PMID: 33637533
- PMCID: PMC7909873
- DOI: 10.1126/sciadv.abe6202
Late-stage stitching enabled by manganese-catalyzed C─H activation: Peptide ligation and access to cyclopeptides
Abstract
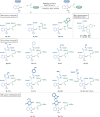
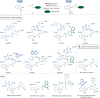
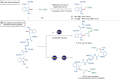
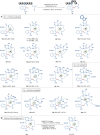
Bioorthogonal late-stage diversification of structurally complex peptides bears enormous potential for drug discovery and molecular imaging. Despite major accomplishments, these strategies heavily rely on noble-metal catalysis. Herein, we report on a manganese(I)-catalyzed peptide C─H hydroarylation that enabled the stitching of peptidic and sugar fragments, under exceedingly mild and racemization-free conditions. This convergent approach represents an atom-economical alternative to traditional iterative peptide synthesis. The robustness of the manganese(I) catalysis regime is reflected by the full tolerance of a plethora of sensitive functional groups. Our strategy enabled an expedient access to challenging cyclic peptides by a modular late-stage macrocyclization of structurally complex peptides.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Blakemore D. C., Castro L., Churcher I., Rees D. C., Thomas A. W., Wilson D. M., Wood A., Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018). - PubMed
-
- Räder A. F. B., Weinmüller M., Reichart F., Schumacher-Klinger A., Merzbach S., Gilon C., Hoffman A., Kessler H., Orally active peptides: Is there a magic bullet? Angew. Chem. Int. Ed. 57, 14414–14438 (2018). - PubMed
-
- Smolyar I. V., Yudin A. K., Nenajdenko V. G., Heteroaryl rings in peptide macrocycles. Chem. Rev. 119, 10032–10240 (2019). - PubMed
-
- Itoh H., Inoue M., Comprehensive structure–activity relationship studies of macrocyclic natural products enabled by their total syntheses. Chem. Rev. 119, 10002–10031 (2019). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

