Succession of the Resident Soil Microbial Community in Response to Periodic Inoculations
- PMID: 33637572
- PMCID: PMC8091015
- DOI: 10.1128/AEM.00046-21
Succession of the Resident Soil Microbial Community in Response to Periodic Inoculations
Abstract
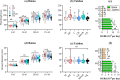
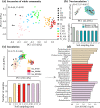
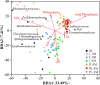
To maintain the beneficial effects of microbial inoculants on plants and soil, repeated inoculation represents a promising option. Until now, the impacts of one-off inoculation on the native microbiome have been explored, but it remains unclear how long and to what extent the periodic inoculations would affect the succession of the resident microbiome in bulk soil. Here, we examined the dynamic responses of plant growth, soil functions, and the resident bacterial community in the bulk soil to periodic inoculations of phosphate-solubilizing and N2-fixing bacteria alone or in combination. Compared to single-strain inoculation, coinoculation better stimulated plant growth and soil nutrients. However, the benefits from inoculants did not increase with repeated inoculations and were not maintained after transplantation to a different site. In response to microbial inoculants, three patterns of shifts in the bacterial composition were observed: fold increase, fold decrease, and resilience. The periodic inoculations impacted the succession course of resident bacterial communities in bulk soil, mainly driven by changes in soil pH and nitrate, resulting in the development of three main cluster types throughout the investigation. The single and mixed inoculants transiently modulated the variation in the resident community in association with soil pH and the C/N ratio, but finally, the community established and showed resilience to subsequent inoculations. Consequently, the necessity of repeated inoculations should be reconsidered, and while the different microbial inoculants showed distinct impacts on resident microbiome succession, the communities ultimately exhibited resilience.IMPORTANCE Introducing beneficial microbes to the plant-soil system is an environmentally friendly approach to improve the crop yield and soil environment. Numerous studies have attempted to reveal the impacts of inoculation on the rhizosphere microbiome. However, little is known about the effectiveness of periodic inoculations on soil functioning. In addition, the long-term impact of repeated inoculations on the native community remains unclear. Here, we track the succession traits of the resident microbiome in the bulk soil across a growing season and identify the taxon clusters that respond differently to periodic inoculation. Crucially, we compare the development of the resident community composition with and without inoculation, thus providing new insight into the interactions between resident microbes and intruders. Finally, we conclude that initial inoculation plays a more important role in influencing the whole system, and the native microbial community exhibits traits of resilience, but no resistance, to the subsequent inoculations.
Keywords: beneficial microorganisms; inoculant type; microbial community succession; periodic inoculation; resident microbiome; soil remediation.
Copyright © 2021 Wang et al.
Figures



References
-
- Thiele-Bruhn S, Bloem J, de Vries FT, Kalbitz K, Wagg C. 2012. Linking soil biodiversity and agricultural soil management. Curr Opin Environ Sustain 4:523–528. 10.1016/j.cosust.2012.06.004. - DOI
-
- Zhong Y, Liu J, Jia X, Shangguan Z, Wang R, Yan W. 2020. Microbial community assembly and metabolic function during wheat straw decomposition under different nitrogen fertilization treatments. Biol Fertil Soils 56:697–710. 10.1007/s00374-020-01438-z. - DOI
-
- Suleiman AKA, Gonzatto R, Aita C, Lupatini M, Jacques RJS, Kuramae EE, Antoniolli ZI, Roesch LFW. 2016. Temporal variability of soil microbial communities after application of dicyandiamide-treated swine slurry and mineral fertilizers. Soil Biol Biochem 97:71–82. 10.1016/j.soilbio.2016.03.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

