Identification of a Diguanylate Cyclase That Facilitates Biofilm Formation on Electrodes by Shewanella oneidensis MR-1
- PMID: 33637573
- PMCID: PMC8091010
- DOI: 10.1128/AEM.00201-21
Identification of a Diguanylate Cyclase That Facilitates Biofilm Formation on Electrodes by Shewanella oneidensis MR-1
Abstract
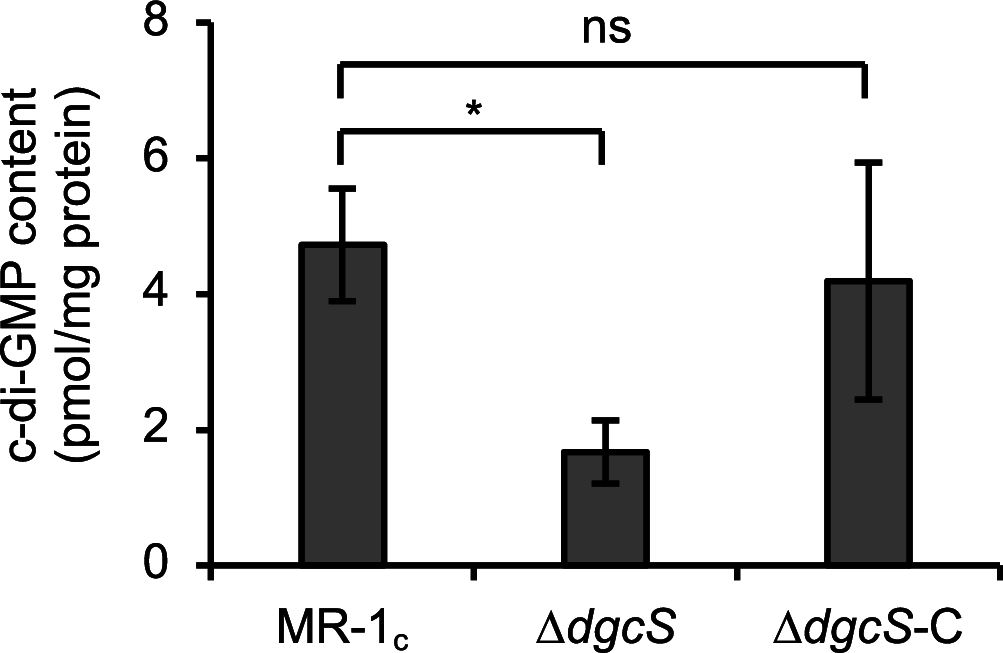
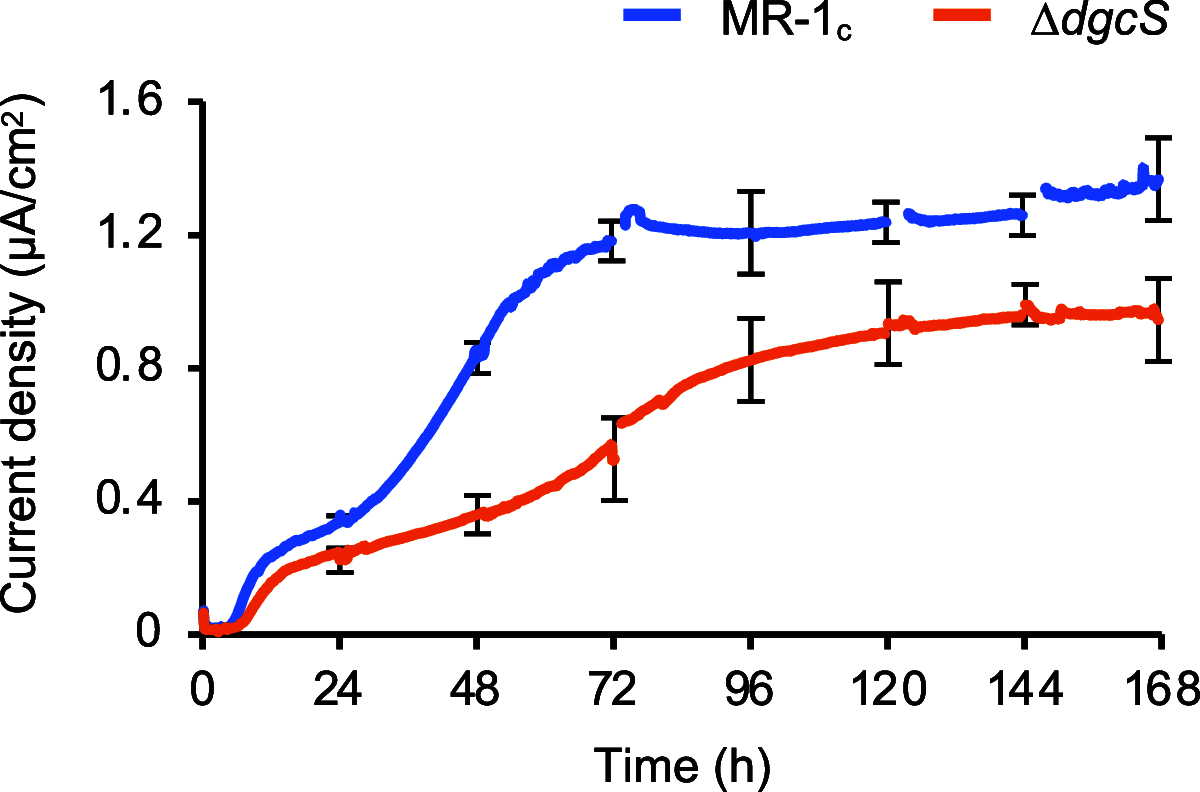
In many bacteria, cyclic diguanosine monophosphate (c-di-GMP), synthesized by diguanylate cyclase (DGC), serves as a second messenger involved in the regulation of biofilm formation. Although studies have suggested that c-di-GMP also regulates the formation of electrochemically active biofilms (EABFs) by Shewanella oneidensis MR-1, DGCs involved in this process remained to be identified. Here, we report that the SO_1646 gene, hereafter named dgcS, is upregulated under medium flow conditions in electrochemical flow cells (EFCs), and its product (DgcS) functions as a major DGC in MR-1. In vitro assays demonstrated that purified DgcS catalyzed the synthesis of c-di-GMP from GTP. Comparisons of intracellular c-di-GMP levels in the wild-type strain and a dgcS deletion mutant (ΔdgcS mutant) showed that production of c-di-GMP was markedly reduced in the ΔdgcS mutant when cells were grown in batch cultures and on electrodes in EFCs. Cultivation of the ΔdgcS mutant in EFCs also revealed that the loss of DgcS resulted in impaired biofilm formation and decreased current generation. These findings demonstrate that MR-1 uses DgcS to synthesize c-di-GMP under medium flow conditions, thereby activating biofilm formation on electrodes.IMPORTANCE Bioelectrochemical systems (BESs) have attracted wide attention owing to their utility in sustainable biotechnology processes, such as microbial fuel cells and electrofermentation systems. In BESs, electrochemically active bacteria (EAB) form biofilms on electrode surfaces, thereby serving as effective catalysts for the interconversion between chemical and electric energy. It is therefore important to understand mechanisms for the formation of biofilm by EAB grown on electrodes. Here, we show that a model EAB, S. oneidensis MR-1, expresses DgcS as a major DGC, thereby activating the formation of biofilms on electrodes via c-di-GMP-dependent signal transduction cascades. The findings presented herein provide the molecular basis for improving electrochemical interactions between EAB and electrodes in BESs. The results also offer molecular insights into how Shewanella regulates biofilm formation on solid surfaces in the natural environment.
Keywords: biofilm; cyclic di-GMP; diguanylate cyclase; electrochemically active bacteria; flow cell.
Copyright © 2021 American Society for Microbiology.
Figures

References
-
- Rabaey K, Angenent L, Schroder U, Keller J (ed). 2009. Bioelectrochemical systems: from extracellular electron transfer to biotechnological application. International Water Association, London, United Kingdom.
-
- Borole AP, Reguera G, Ringeisen B, Wang Z-W, Feng Y, Kim BH. 2011. Electroactive biofilms: current status and future research needs. Energy Environ Sci 4:4813–4813. 10.1039/c1ee02511b. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

