Site-Specific and Residualizing Linker for 18F Labeling with Enhanced Renal Clearance: Application to an Anti-HER2 Single-Domain Antibody Fragment
- PMID: 33637584
- PMCID: PMC8612331
- DOI: 10.2967/jnumed.120.261446
Site-Specific and Residualizing Linker for 18F Labeling with Enhanced Renal Clearance: Application to an Anti-HER2 Single-Domain Antibody Fragment
Abstract
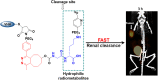
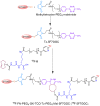
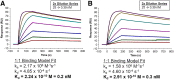
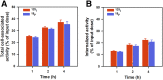
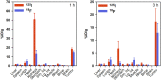
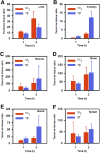
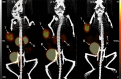
Single-domain antibody fragments (sdAbs) are promising vectors for immuno-PET; however, better methods for labeling sdAbs with 18F are needed. Herein, we evaluate a site-specific strategy using an 18F residualizing motif and the anti-epidermal growth factor receptor 2 (HER2) sdAb 5F7 bearing an engineered C-terminal GGC tail (5F7GGC). Methods: 5F7GGC was site-specifically attached with a tetrazine-bearing agent via thiol-maleimide reaction. The resultant conjugate was labeled with 18F by inverse electron demand Diels-Alder cycloaddition with a trans-cyclooctene attached to 6-18F-fluoronicotinoyl moiety via a renal brush border enzyme-cleavable linker and a PEG4 chain (18F-5F7GGC). For comparisons, 5F7 sdAb was labeled using the prototypical residualizing agent, N-succinimidyl 3-(guanidinomethyl)-5-125I-iodobenzoate (iso-125I-SGMIB). The 2 labeled sdAbs were compared in paired-label studies performed in the HER2-expressing BT474M1 breast carcinoma cell line and athymic mice bearing BT474M1 subcutaneous xenografts. Small-animal PET/CT imaging after administration of 18F-5F7GGC in the above mouse model was also performed. Results:18F-5F7GGC was synthesized in an overall radiochemical yield of 8.9% ± 3.2% with retention of HER2 binding affinity and immunoreactivity. The total cell-associated and intracellular activity for 18F-5F7GGC was similar to that for coincubated iso-125I-SGMIB-5F7. Likewise, the uptake of 18F-5F7GGC in BT474M1 xenografts in mice was similar to that for iso-125I-SGMIB-5F7; however, 18F-5F7GGC exhibited significantly more rapid clearance from the kidney. Small-animal PET/CT imaging confirmed high uptake and retention in the tumor with very little background activity at 3 h except in the bladder. Conclusion: This site-specific and residualizing 18F-labeling strategy could facilitate clinical translation of 5F7 anti-HER2 sdAb as well as other sdAbs for immuno-PET.
Keywords: HER2; click chemistry; immuno-PET; single-domain antibody fragment; site-specific labeling.
© 2021 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
References
-
- Martin V, Cappuzzo F, Mazzucchelli L, Frattini M. HER2 in solid tumors: more than 10 years under the microscope—where are we now? Future Oncol. 2014;10:1469–1486. - PubMed
-
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, McShane LM, Dowsett M. HER2 testing in breast cancer: American Society of Clinical Oncology/College of AMERICAN Pathologists clinical practice guideline focused update summary. J Oncol Pract. 2018;14:437–441. - PubMed
-
- Xavier C, Vaneycken I, D’Huyvetter M, et al. . Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013;54:776–784. - PubMed
-
- Morris O, Fairclough M, Grigg J, Prenant C, McMahon A. A review of approaches to 18F radiolabelling affinity peptides and proteins. J Labelled Comp Radiopharm. 2019;62:4–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
