SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction
- PMID: 33637603
- PMCID: PMC8139673
- DOI: 10.1128/JVI.00110-21
SARS-CoV-2 causes severe epithelial inflammation and barrier dysfunction
Abstract
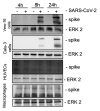
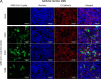
Infections with SARS-CoV-2 can be asymptomatic, but they can also be accompanied by a variety of symptoms that result in mild to severe coronavirus disease-19 (COVID-19) and are sometimes associated with systemic symptoms. Although the viral infection originates in the respiratory system, it is unclear how the virus can overcome the alveolar barrier, which is observed in severe COVID-19 disease courses. To elucidate the viral effects on the barrier integrity and immune reactions, we used mono-cell culture systems and a complex human chip model composed of epithelial, endothelial, and mononuclear cells. Our data show that SARS-CoV-2 efficiently infected epithelial cells with high viral loads and inflammatory response, including interferon expression. By contrast, the adjacent endothelial layer was neither infected nor did it show productive virus replication or interferon release. With prolonged infection, both cell types were damaged, and the barrier function was deteriorated, allowing the viral particles to overbear. In our study, we demonstrate that although SARS-CoV-2 is dependent on the epithelium for efficient replication, the neighboring endothelial cells are affected, e.g., by the epithelial cytokines or components induced during infection, which further results in the damage of the epithelial/endothelial barrier function and viral dissemination.IMPORTANCESARS-CoV-2 challenges healthcare systems and societies worldwide in unprecedented ways. Although numerous new studies have been conducted, research to better understand the molecular pathogen-host interactions are urgently needed. For this, experimental models have to be developed and adapted. In the present study we used mono cell-culture systems and we established a complex chip model, where epithelial and endothelial cells are cultured in close proximity. We demonstrate that epithelial cells can be infected with SARS-CoV-2, while the endothelium did not show any infection signs. Since SARS-CoV-2 is able to establish viremia, the link to thromboembolic events in severe COVID-19 courses is evident. However, whether the endothelial layer is damaged by the viral pathogens or whether other endothelial-independent homeostatic factors are induced by the virus is essential for understanding the disease development. Therefore, our study is important as it demonstrates that the endothelial layer could not be infected by SARS-CoV-2 in our in vitro experiments, but we were able to show the destruction of the epithelial-endothelial barrier in our chip model. From our experiments we can assume that virus-induced host factors disturbed the epithelial-endothelial barrier function and thereby promote viral spread.
Copyright © 2021 Deinhardt-Emmer et al.
Figures



References
-
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. 2020. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infectious Diseases 20:1135–1140. 10.1016/S1473-3099(20)30434-5. - DOI - PMC - PubMed
-
- Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen H-R, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. 2020. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 173:268–277. 10.7326/M20-2003. - DOI - PMC - PubMed
-
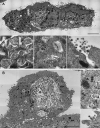
- Deinhardt-Emmer S, Wittschieber D, Sanft J, Kleemann S, Elschner S, Haupt KF, Vau V, Häring C, Rödel J, Henke A, Ehrhardt C, Bauer M, Philipp M, Gaßler N, Nietzsche S, Löffler B, Mall G. 2020. Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and correlation to tissue damage. bioRxiv 10.1101/2020.07.01.182550:2020.07.01.182550. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

