The crosstalk between HIFs and mitochondrial dysfunctions in cancer development
- PMID: 33637686
- PMCID: PMC7910460
- DOI: 10.1038/s41419-021-03505-1
The crosstalk between HIFs and mitochondrial dysfunctions in cancer development
Abstract
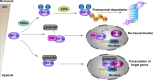
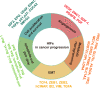
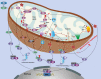
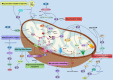
Mitochondria are essential cellular organelles that are involved in regulating cellular energy, metabolism, survival, and proliferation. To some extent, cancer is a genetic and metabolic disease that is closely associated with mitochondrial dysfunction. Hypoxia-inducible factors (HIFs), which are major molecules that respond to hypoxia, play important roles in cancer development by participating in multiple processes, such as metabolism, proliferation, and angiogenesis. The Warburg phenomenon reflects a pseudo-hypoxic state that activates HIF-1α. In addition, a product of the Warburg effect, lactate, also induces HIF-1α. However, Warburg proposed that aerobic glycolysis occurs due to a defect in mitochondria. Moreover, both HIFs and mitochondrial dysfunction can lead to complex reprogramming of energy metabolism, including reduced mitochondrial oxidative metabolism, increased glucose uptake, and enhanced anaerobic glycolysis. Thus, there may be a connection between HIFs and mitochondrial dysfunction. In this review, we systematically discuss the crosstalk between HIFs and mitochondrial dysfunctions in cancer development. Above all, the stability and activity of HIFs are closely influenced by mitochondrial dysfunction related to tricarboxylic acid cycle, electron transport chain components, mitochondrial respiration, and mitochondrial-related proteins. Furthermore, activation of HIFs can lead to mitochondrial dysfunction by affecting multiple mitochondrial functions, including mitochondrial oxidative capacity, biogenesis, apoptosis, fission, and autophagy. In general, the regulation of tumorigenesis and development by HIFs and mitochondrial dysfunction are part of an extensive and cooperative network.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

