Review of biosensing with whispering-gallery mode lasers
- PMID: 33637696
- PMCID: PMC7910454
- DOI: 10.1038/s41377-021-00471-3
Review of biosensing with whispering-gallery mode lasers
Abstract
Lasers are the pillars of modern optics and sensing. Microlasers based on whispering-gallery modes (WGMs) are miniature in size and have excellent lasing characteristics suitable for biosensing. WGM lasers have been used for label-free detection of single virus particles, detection of molecular electrostatic changes at biointerfaces, and barcode-type live-cell tagging and tracking. The most recent advances in biosensing with WGM microlasers are described in this review. We cover the basic concepts of WGM resonators, the integration of gain media into various active WGM sensors and devices, and the cutting-edge advances in photonic devices for micro- and nanoprobing of biological samples that can be integrated with WGM lasers.
Conflict of interest statement
The authors declare that they have no conflict of interest. All figures were reproduced with corresponding permissions provided by publishers.
Figures


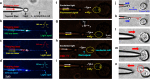

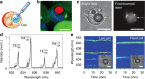


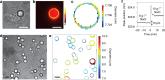
References
-
- Maiman TH. Stimulated optical radiation in ruby. Nature. 1960;187:493–494. doi: 10.1038/187493a0. - DOI
-
- Glauber RJ. The quantum theory of optical coherence. Phys. Rev. 1963;130:2529–2539. doi: 10.1103/PhysRev.130.2529. - DOI
-
- Ready, J. F. Industrial Applications of Lasers. 2nd edn (Academic Press, London, 1997).
-
- Schubert M, et al. Monitoring contractility in cardiac tissue with cellular resolution using biointegrated microlasers. Nat. Photonics. 2020;14:452–458. doi: 10.1038/s41566-020-0631-z. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

