Marine furanocembranoids-inspired macrocycles enabled by Pd-catalyzed unactivated C(sp3)-H olefination mediated by donor/donor carbenes
- PMID: 33637703
- PMCID: PMC7910576
- DOI: 10.1038/s41467-021-21484-x
Marine furanocembranoids-inspired macrocycles enabled by Pd-catalyzed unactivated C(sp3)-H olefination mediated by donor/donor carbenes
Abstract
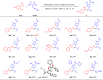
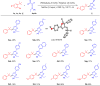
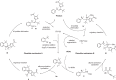
Biomimetic modularization and function-oriented synthesis of structurally diversified natural product-like macrocycles in a step-economical fashion is highly desirable. Inspired by marine furanocembranoids, herein, we synthesize diverse alkenes substituted furan-embedded macrolactams via a modular biomimetic assembly strategy. The success of this assembly is the development of crucial Pd-catalyzed carbene coupling between ene-yne-ketones as donor/donor carbene precursors and unactivated Csp3‒H bonds which represents a great challenge in organic synthesis. Notably, this method not only obviates the use of unstable, explosive, and toxic diazo compounds, but also can be amenable to allenyl ketones carbene precursors. DFT calculations demonstrate that a formal 1,4-Pd shift could be involved in the mechanism. Moreover, the collected furanocembranoids-like macrolactams show significant anti-inflammatory activities against TNF-α, IL-6, and IL-1β and the cytotoxicity is comparable to Dexamethasone.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Cu(I)-catalyzed cross-coupling of conjugated ene-yne-ketones and terminal alkynes: synthesis of furan-substituted allenes.Org Lett. 2014 Aug 15;16(16):4082-5. doi: 10.1021/ol501747f. Epub 2014 Jul 30. Org Lett. 2014. PMID: 25075633
-
Pd-catalyzed cross-coupling of terminal alkynes with ene-yne-ketones: access to conjugated enynes via metal carbene migratory insertion.Chem Commun (Camb). 2015 Jun 30;51(56):11233-5. doi: 10.1039/c5cc03559g. Chem Commun (Camb). 2015. PMID: 26077197
-
Manganese-Catalyzed Redox-Neutral C-H Olefination of Ketones with Unactivated Alkenes.Angew Chem Int Ed Engl. 2018 Sep 10;57(37):12071-12075. doi: 10.1002/anie.201806287. Epub 2018 Aug 20. Angew Chem Int Ed Engl. 2018. PMID: 30035848
-
Recent progress on donor and donor-donor carbenes.Chem Soc Rev. 2020 Feb 10;49(3):908-950. doi: 10.1039/c9cs00542k. Chem Soc Rev. 2020. PMID: 31958107 Review.
-
Co(III), Rh(III) & Ir(III)-Catalyzed Direct C-H Alkylation/Alkenylation/Arylation with Carbene Precursors.Chem Asian J. 2021 Mar 1;16(5):443-459. doi: 10.1002/asia.202001219. Epub 2021 Feb 4. Chem Asian J. 2021. PMID: 33448653 Review.
References
-
- Newman, D. J., Cragg, G. M., Macrocycles in Drug Discovery, Vol. 40 (ed J. Levin) 1–36 (The Royal Society of Chemistry, Cambridge, 2015).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

