Functional regeneration and repair of tendons using biomimetic scaffolds loaded with recombinant periostin
- PMID: 33637721
- PMCID: PMC7910464
- DOI: 10.1038/s41467-021-21545-1
Functional regeneration and repair of tendons using biomimetic scaffolds loaded with recombinant periostin
Abstract
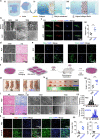
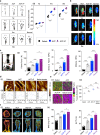
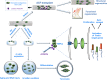
Tendon injuries disrupt the balance between stability and mobility, causing compromised functions and disabilities. The regeneration of mature, functional tendons remains a clinical challenge. Here, we perform transcriptional profiling of tendon developmental processes to show that the extracellular matrix-associated protein periostin (Postn) contributes to the maintenance of tendon stem/progenitor cell (TSPC) functions and promotes tendon regeneration. We show that recombinant periostin (rPOSTN) promotes the proliferation and stemness of TSPCs, and maintains the tenogenic potentials of TSPCs in vitro. We also find that rPOSTN protects TSPCs against functional impairment during long-term passage in vitro. For in vivo tendon formation, we construct a biomimetic parallel-aligned collagen scaffold to facilitate TSPC tenogenesis. Using a rat full-cut Achilles tendon defect model, we demonstrate that scaffolds loaded with rPOSTN promote endogenous TSPC recruitment, tendon regeneration and repair with native-like hierarchically organized collagen fibers. Moreover, newly regenerated tendons show recovery of mechanical properties and locomotion functions.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
An asymmetric chitosan scaffold for tendon tissue engineering: In vitro and in vivo evaluation with rat tendon stem/progenitor cells.Acta Biomater. 2018 Jun;73:377-387. doi: 10.1016/j.actbio.2018.04.027. Epub 2018 Apr 17. Acta Biomater. 2018. PMID: 29678676
-
An all-silk-based functional system promotes tendon regeneration by regulating the cell fate of TSPCs in an inflammatory microenvironment.Acta Biomater. 2025 Jun 15;200:432-451. doi: 10.1016/j.actbio.2025.05.040. Epub 2025 May 26. Acta Biomater. 2025. PMID: 40409996
-
Rejuvenation of tendon stem/progenitor cells for functional tendon regeneration through platelet-derived exosomes loaded with recombinant Yap1.Acta Biomater. 2023 Apr 15;161:80-99. doi: 10.1016/j.actbio.2023.02.018. Epub 2023 Feb 17. Acta Biomater. 2023. PMID: 36804538
-
From Tendon Injury to Collagen-based Tendon Regeneration: Overview and Recent Advances.Curr Pharm Des. 2017;23(24):3483-3506. doi: 10.2174/1381612823666170516130515. Curr Pharm Des. 2017. PMID: 28521693 Review.
-
Mesenchymal stem cells in tendon repair and regeneration: basic understanding and translational challenges.Ann N Y Acad Sci. 2016 Nov;1383(1):88-96. doi: 10.1111/nyas.13262. Epub 2016 Oct 5. Ann N Y Acad Sci. 2016. PMID: 27706825 Review.
Cited by
-
Mechanical loading is required for initiation of extracellular matrix deposition at the developing murine myotendinous junction.Matrix Biol. 2023 Feb;116:28-48. doi: 10.1016/j.matbio.2023.01.003. Epub 2023 Jan 26. Matrix Biol. 2023. PMID: 36709857 Free PMC article.
-
Energy-Supporting Enzyme-Mimic Nanoscaffold Facilitates Tendon Regeneration Based on a Mitochondrial Protection and Microenvironment Remodeling Strategy.Adv Sci (Weinh). 2022 Nov;9(31):e2202542. doi: 10.1002/advs.202202542. Epub 2022 Aug 24. Adv Sci (Weinh). 2022. PMID: 36000796 Free PMC article.
-
Current progress in growth factors and extracellular vesicles in tendon healing.Int Wound J. 2023 Nov;20(9):3871-3883. doi: 10.1111/iwj.14261. Epub 2023 Jun 8. Int Wound J. 2023. PMID: 37291064 Free PMC article. Review.
-
Mesoporous Particle Embedded Nanofibrous Scaffolds Sustain Biological Factors for Tendon Tissue Engineering.ACS Mater Au. 2023 Jul 24;3(6):636-645. doi: 10.1021/acsmaterialsau.3c00012. eCollection 2023 Nov 8. ACS Mater Au. 2023. PMID: 38089667 Free PMC article.
-
TRIM54 alleviates inflammation and apoptosis by stabilizing YOD1 in rat tendon-derived stem cells.J Biol Chem. 2024 Jan;300(1):105510. doi: 10.1016/j.jbc.2023.105510. Epub 2023 Nov 30. J Biol Chem. 2024. PMID: 38042492 Free PMC article.
References
-
- Sharma, P. & Maffulli, N. Tendon injury and tendinopathy: healing and repair. J. Bone. Joint. Surg. Am.87, 187–202 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

