Maintenance of type 2 glycolytic myofibers with age by Mib1-Actn3 axis
- PMID: 33637766
- PMCID: PMC7910585
- DOI: 10.1038/s41467-021-21621-6
Maintenance of type 2 glycolytic myofibers with age by Mib1-Actn3 axis
Abstract
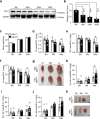
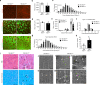
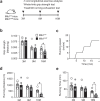
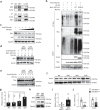
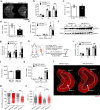
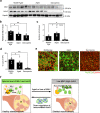
Age-associated muscle atrophy is a debilitating condition associated with loss of muscle mass and function with age that contributes to limitation of mobility and locomotion. However, the underlying mechanisms of how intrinsic muscle changes with age are largely unknown. Here we report that, with age, Mind bomb-1 (Mib1) plays important role in skeletal muscle maintenance via proteasomal degradation-dependent regulation of α-actinin 3 (Actn3). The disruption of Mib1 in myofibers (Mib1ΔMF) results in alteration of type 2 glycolytic myofibers, muscle atrophy, impaired muscle function, and Actn3 accumulation. After chronic exercise, Mib1ΔMF mice show muscle atrophy even at young age. However, when Actn3 level is downregulated, chronic exercise-induced muscle atrophy is ameliorated. Importantly, the Mib1 and Actn3 levels show clinical relevance in human skeletal muscles accompanied by decrease in skeletal muscle function with age. Together, these findings reveal the significance of the Mib1-Actn3 axis in skeletal muscle maintenance with age and suggest the therapeutic potential for the treatment or amelioration of age-related muscle atrophy.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The effect of α-actinin-3 deficiency on muscle aging.Exp Gerontol. 2011 Apr;46(4):292-302. doi: 10.1016/j.exger.2010.11.006. Epub 2010 Nov 26. Exp Gerontol. 2011. PMID: 21112313
-
Readthrough of ACTN3 577X nonsense mutation produces full-length α-actinin-3 protein.Biochem Biophys Res Commun. 2018 Jul 20;502(3):422-428. doi: 10.1016/j.bbrc.2018.05.193. Epub 2018 May 30. Biochem Biophys Res Commun. 2018. PMID: 29857001
-
Differential regulation of Actn2 and Actn3 expression during unfolded protein response in C2C12 myotubes.J Muscle Res Cell Motil. 2020 Sep;41(2-3):199-209. doi: 10.1007/s10974-020-09582-7. Epub 2020 May 25. J Muscle Res Cell Motil. 2020. PMID: 32451822
-
Is evolutionary loss our gain? The role of ACTN3 p.Arg577Ter (R577X) genotype in athletic performance, ageing, and disease.Hum Mutat. 2018 Dec;39(12):1774-1787. doi: 10.1002/humu.23663. Epub 2018 Nov 8. Hum Mutat. 2018. PMID: 30281865 Review.
-
More than a 'speed gene': ACTN3 R577X genotype, trainability, muscle damage, and the risk for injuries.Eur J Appl Physiol. 2019 Jan;119(1):49-60. doi: 10.1007/s00421-018-4010-0. Epub 2018 Oct 16. Eur J Appl Physiol. 2019. PMID: 30327870 Review.
Cited by
-
Methylome-proteome integration after late-life voluntary exercise training reveals regulation and target information for improved skeletal muscle health.J Physiol. 2025 Jan;603(1):211-237. doi: 10.1113/JP286681. Epub 2024 Jul 26. J Physiol. 2025. PMID: 39058663 Free PMC article.
-
Function and Molecular Mechanism of Circhomer1 in Myogenesis.Int J Mol Sci. 2025 Jun 28;26(13):6264. doi: 10.3390/ijms26136264. Int J Mol Sci. 2025. PMID: 40650039 Free PMC article.
-
Epigenetics of Skeletal Muscle Atrophy.Int J Mol Sci. 2024 Jul 31;25(15):8362. doi: 10.3390/ijms25158362. Int J Mol Sci. 2024. PMID: 39125931 Free PMC article. Review.
-
Mll4 in skeletal muscle fibers maintains muscle stem cells.Skelet Muscle. 2024 Dec 23;14(1):35. doi: 10.1186/s13395-024-00369-9. Skelet Muscle. 2024. PMID: 39710699 Free PMC article.
-
CLIC5 promotes myoblast differentiation and skeletal muscle regeneration via the BGN-mediated canonical Wnt/β-catenin signaling pathway.Sci Adv. 2024 Oct 11;10(41):eadq6795. doi: 10.1126/sciadv.adq6795. Epub 2024 Oct 11. Sci Adv. 2024. PMID: 39999205 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

