A plant plasma-membrane H+-ATPase promotes yeast TORC1 activation via its carboxy-terminal tail
- PMID: 33637787
- PMCID: PMC7910539
- DOI: 10.1038/s41598-021-83525-1
A plant plasma-membrane H+-ATPase promotes yeast TORC1 activation via its carboxy-terminal tail
Abstract
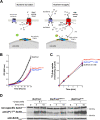
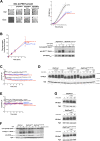
The Target of Rapamycin Complex 1 (TORC1) involved in coordination of cell growth and metabolism is highly conserved among eukaryotes. Yet the signals and mechanisms controlling its activity differ among taxa, according to their biological specificities. A common feature of fungal and plant cells, distinguishing them from animal cells, is that their plasma membrane contains a highly abundant H+-ATPase which establishes an electrochemical H+ gradient driving active nutrient transport. We have previously reported that in yeast, nutrient-uptake-coupled H+ influx elicits transient TORC1 activation and that the plasma-membrane H+-ATPase Pma1 plays an important role in this activation, involving more than just establishment of the H+ gradient. We show here that the PMA2 H+-ATPase from the plant Nicotiana plumbaginifolia can substitute for Pma1 in yeast, to promote H+-elicited TORC1 activation. This H+-ATPase is highly similar to Pma1 but has a longer carboxy-terminal tail binding 14-3-3 proteins. We report that a C-terminally truncated PMA2, which remains fully active, fails to promote H+-elicited TORC1 activation. Activation is also impaired when binding of PMA2 to 14-3-3 s is hindered. Our results show that at least some plant plasma-membrane H+-ATPases share with yeast Pma1 the ability to promote TORC1 activation in yeast upon H+-coupled nutrient uptake.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Plasma membrane H+-ATPases promote TORC1 activation in plant suspension cells.iScience. 2022 Apr 11;25(5):104238. doi: 10.1016/j.isci.2022.104238. eCollection 2022 May 20. iScience. 2022. PMID: 35494253 Free PMC article.
-
Phosphoregulation of the yeast Pma1 H+-ATPase autoinhibitory domain involves the Ptk1/2 kinases and the Glc7 PP1 phosphatase and is under TORC1 control.PLoS Genet. 2024 Jan 16;20(1):e1011121. doi: 10.1371/journal.pgen.1011121. eCollection 2024 Jan. PLoS Genet. 2024. PMID: 38227612 Free PMC article.
-
The yeast H+-ATPase Pma1 promotes Rag/Gtr-dependent TORC1 activation in response to H+-coupled nutrient uptake.Elife. 2018 Mar 23;7:e31981. doi: 10.7554/eLife.31981. Elife. 2018. PMID: 29570051 Free PMC article.
-
Lipid-dependent surface transport of the proton pumping ATPase: a model to study plasma membrane biogenesis in yeast.Biochimie. 2007 Feb;89(2):249-54. doi: 10.1016/j.biochi.2006.07.020. Epub 2006 Aug 15. Biochimie. 2007. PMID: 16938383 Review.
-
Plasma membrane ATPase of fungi and plants as a novel type of proton pump.Curr Top Cell Regul. 1984;23:87-126. doi: 10.1016/b978-0-12-152823-2.50007-6. Curr Top Cell Regul. 1984. PMID: 6327194 Review. No abstract available.
Cited by
-
Pib2 as an Emerging Master Regulator of Yeast TORC1.Biomolecules. 2021 Oct 9;11(10):1489. doi: 10.3390/biom11101489. Biomolecules. 2021. PMID: 34680122 Free PMC article. Review.
-
Plasma membrane H+-ATPases promote TORC1 activation in plant suspension cells.iScience. 2022 Apr 11;25(5):104238. doi: 10.1016/j.isci.2022.104238. eCollection 2022 May 20. iScience. 2022. PMID: 35494253 Free PMC article.
-
Dual RNA-Seq Analysis Pinpoints a Balanced Regulation between Symbiosis and Immunity in Medicago truncatula-Sinorhizobium meliloti Symbiotic Nodules.Int J Mol Sci. 2023 Nov 10;24(22):16178. doi: 10.3390/ijms242216178. Int J Mol Sci. 2023. PMID: 38003367 Free PMC article.
-
Phosphoregulation of the yeast Pma1 H+-ATPase autoinhibitory domain involves the Ptk1/2 kinases and the Glc7 PP1 phosphatase and is under TORC1 control.PLoS Genet. 2024 Jan 16;20(1):e1011121. doi: 10.1371/journal.pgen.1011121. eCollection 2024 Jan. PLoS Genet. 2024. PMID: 38227612 Free PMC article.
-
Regulatory dynamics of Sch9 in response to cytosolic acidification: From spatial reconfiguration to cellular adaptation to stresses.iScience. 2024 Dec 10;28(1):111573. doi: 10.1016/j.isci.2024.111573. eCollection 2025 Jan 17. iScience. 2024. PMID: 39811664 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

