Diffusion histology imaging differentiates distinct pediatric brain tumor histology
- PMID: 33637807
- PMCID: PMC7910493
- DOI: 10.1038/s41598-021-84252-3
Diffusion histology imaging differentiates distinct pediatric brain tumor histology
Abstract
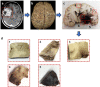
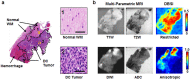
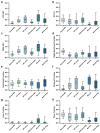
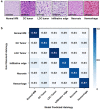
High-grade pediatric brain tumors exhibit the highest cancer mortality rates in children. While conventional MRI has been widely adopted for examining pediatric high-grade brain tumors clinically, accurate neuroimaging detection and differentiation of tumor histopathology for improved diagnosis, surgical planning, and treatment evaluation, remains an unmet need in their clinical management. We employed a novel Diffusion Histology Imaging (DHI) approach employing diffusion basis spectrum imaging (DBSI) derived metrics as the input classifiers for deep neural network analysis. DHI aims to detect, differentiate, and quantify heterogeneous areas in pediatric high-grade brain tumors, which include normal white matter (WM), densely cellular tumor, less densely cellular tumor, infiltrating edge, necrosis, and hemorrhage. Distinct diffusion metric combination would thus indicate the unique distributions of each distinct tumor histology features. DHI, by incorporating DBSI metrics and the deep neural network algorithm, classified pediatric tumor histology with an overall accuracy of 85.8%. Receiver operating analysis (ROC) analysis suggested DHI's great capability in distinguishing individual tumor histology with AUC values (95% CI) of 0.984 (0.982-0.986), 0.960 (0.956-0.963), 0.991 (0.990-0.993), 0.950 (0.944-0.956), 0.977 (0.973-0.981) and 0.976 (0.972-0.979) for normal WM, densely cellular tumor, less densely cellular tumor, infiltrating edge, necrosis and hemorrhage, respectively. Our results suggest that DBSI-DNN, or DHI, accurately characterized and classified multiple tumor histologic features in pediatric high-grade brain tumors. If these results could be further validated in patients, the novel DHI might emerge as a favorable alternative to the current neuroimaging techniques to better guide biopsy and resection as well as monitor therapeutic response in patients with high-grade brain tumors.
Conflict of interest statement
S.K.S. has a financial [ownership] interest in CancerVision LLC and may financially benefit if the company is successful in marketing its product(s) that is/are related to this research. Other authors declared no competing interests.
Figures

Similar articles
-
Diffusion Histology Imaging Combining Diffusion Basis Spectrum Imaging (DBSI) and Machine Learning Improves Detection and Classification of Glioblastoma Pathology.Clin Cancer Res. 2020 Oct 15;26(20):5388-5399. doi: 10.1158/1078-0432.CCR-20-0736. Epub 2020 Jul 21. Clin Cancer Res. 2020. PMID: 32694155 Free PMC article.
-
Intravoxel Incoherent Motion MR Imaging of Pediatric Intracranial Tumors: Correlation with Histology and Diagnostic Utility.AJNR Am J Neuroradiol. 2019 May;40(5):878-884. doi: 10.3174/ajnr.A6052. Epub 2019 Apr 25. AJNR Am J Neuroradiol. 2019. PMID: 31023663 Free PMC article.
-
Multiparametric Analysis of Permeability and ADC Histogram Metrics for Classification of Pediatric Brain Tumors by Tumor Grade.AJNR Am J Neuroradiol. 2018 Mar;39(3):552-557. doi: 10.3174/ajnr.A5502. Epub 2018 Jan 4. AJNR Am J Neuroradiol. 2018. PMID: 29301780 Free PMC article.
-
Differentiation of Low- and High-Grade Gliomas Using High b-Value Diffusion Imaging with a Non-Gaussian Diffusion Model.AJNR Am J Neuroradiol. 2016 Sep;37(9):1643-9. doi: 10.3174/ajnr.A4836. Epub 2016 Jun 2. AJNR Am J Neuroradiol. 2016. PMID: 27256851 Free PMC article.
-
High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery.Neuro Oncol. 2015 Sep;17(9):1199-209. doi: 10.1093/neuonc/nov113. Epub 2015 Jun 27. Neuro Oncol. 2015. PMID: 26117712 Free PMC article. Review.
Cited by
-
Standard clinical approaches and emerging modalities for glioblastoma imaging.Neurooncol Adv. 2022 May 26;4(1):vdac080. doi: 10.1093/noajnl/vdac080. eCollection 2022 Jan-Dec. Neurooncol Adv. 2022. PMID: 35821676 Free PMC article. Review.
-
Artificial intelligence applications in pediatric oncology diagnosis.Explor Target Antitumor Ther. 2023;4(1):157-169. doi: 10.37349/etat.2023.00127. Epub 2023 Feb 28. Explor Target Antitumor Ther. 2023. PMID: 36937318 Free PMC article. Review.
-
Cancerous and Non-Cancerous MRI Classification Using Dual DCNN Approach.Bioengineering (Basel). 2024 Apr 23;11(5):410. doi: 10.3390/bioengineering11050410. Bioengineering (Basel). 2024. PMID: 38790279 Free PMC article.
-
Radio-pathomic approaches in pediatric neuro-oncology: Opportunities and challenges.Neurooncol Adv. 2023 Sep 13;5(1):vdad119. doi: 10.1093/noajnl/vdad119. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37841693 Free PMC article. Review.
-
Quantitative and longitudinal assessment of human placental inflammation using diffusion basis spectrum imaging.NPJ Womens Health. 2025;3(1):1. doi: 10.1038/s44294-024-00049-5. Epub 2025 Jan 3. NPJ Womens Health. 2025. PMID: 39759173 Free PMC article.
References
-
- Curtin, S. C., Minino, A. M. & Anderson, R. N. Declines in cancer death rates among children and adolescents in the United States, 1999–2014. NCHS Data Brief 1–8 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

