Autophagy-enhancing drugs limit mucosal HIV-1 acquisition and suppress viral replication ex vivo
- PMID: 33637808
- PMCID: PMC7910550
- DOI: 10.1038/s41598-021-84081-4
Autophagy-enhancing drugs limit mucosal HIV-1 acquisition and suppress viral replication ex vivo
Abstract
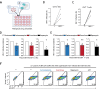
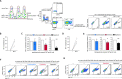
Current direct-acting antiviral therapies are highly effective in suppressing HIV-1 replication. However, mucosal inflammation undermines prophylactic treatment efficacy, and HIV-1 persists in long-lived tissue-derived dendritic cells (DCs) and CD4+ T cells of treated patients. Host-directed strategies are an emerging therapeutic approach to improve therapy outcomes in infectious diseases. Autophagy functions as an innate antiviral mechanism by degrading viruses in specialized vesicles. Here, we investigated the impact of pharmaceutically enhancing autophagy on HIV-1 acquisition and viral replication. To this end, we developed a human tissue infection model permitting concurrent analysis of HIV-1 cellular targets ex vivo. Prophylactic treatment with autophagy-enhancing drugs carbamazepine and everolimus promoted HIV-1 restriction in skin-derived CD11c+ DCs and CD4+ T cells. Everolimus also decreased HIV-1 susceptibility to lab-adapted and transmitted/founder HIV-1 strains, and in vaginal Langerhans cells. Notably, we observed cell-specific effects of therapeutic treatment. Therapeutic rapamycin treatment suppressed HIV-1 replication in tissue-derived CD11c+ DCs, while all selected drugs limited viral replication in CD4+ T cells. Strikingly, both prophylactic and therapeutic treatment with everolimus or rapamycin reduced intestinal HIV-1 productive infection. Our findings highlight host autophagy pathways as an emerging target for HIV-1 therapies, and underscore the relevancy of repurposing clinically-approved autophagy drugs to suppress mucosal HIV-1 replication.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- HIV/AIDS Factsheet. World health organization.https://www.who.int/news-room/fact-sheets/detail/hiv-aids (2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

