Influence of bound dodecanoic acid on the reconstitution of albumin nanoparticles from a lyophilized state
- PMID: 33637809
- PMCID: PMC7910568
- DOI: 10.1038/s41598-021-84131-x
Influence of bound dodecanoic acid on the reconstitution of albumin nanoparticles from a lyophilized state
Abstract
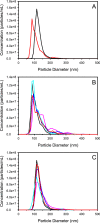
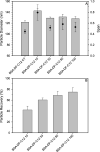
The development of reference standards for nanoparticle sizing allows for cross laboratory studies and effective transfer of particle sizing methodology. To facilitate this, these reference standards must be stable upon long-term storage. Here, we examine factors that influence the properties of cross-linked albumin nanoparticles, fabricated with an ethanol desolvation method, when reconstituted from a lyophilized state. We demonstrate, with nanoparticle tracking analysis, no significant changes in mean particle diameter upon reconstitution of albumin nanoparticles fabricated with bovine serum albumin loaded with dodecanoic acid, when compared to nanoparticles fabricated with a fatty acid-free BSA. We attribute this stability to the modulation of nanoparticle charge-charge interactions at dodecanoic acid specific binding locations. Furthermore, we demonstrate this in a lyophilized state over six months when stored at - 80 °C. We also show that the reconstitution process is readily transferable between technicians and laboratories and further confirm our finding with dynamic light scattering analysis.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Nanoparticle size and production efficiency are affected by the presence of fatty acids during albumin nanoparticle fabrication.PLoS One. 2017 Dec 27;12(12):e0189814. doi: 10.1371/journal.pone.0189814. eCollection 2017. PLoS One. 2017. PMID: 29281685 Free PMC article.
-
Preparation of Bovine Serum Albumin (BSA) nanoparticles by desolvation using a membrane contactor: a new tool for large scale production.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):398-405. doi: 10.1016/j.ejpb.2013.06.014. Epub 2013 Jun 27. Eur J Pharm Biopharm. 2013. PMID: 23811438
-
Robust size control of bovine serum albumin (BSA) nanoparticles by intermittent addition of a desolvating agent and the particle formation mechanism.Food Chem. 2013 Nov 15;141(2):695-701. doi: 10.1016/j.foodchem.2013.04.059. Epub 2013 Apr 29. Food Chem. 2013. PMID: 23790836
-
Lyophilization and stability of antibody-conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery.Int J Nanomedicine. 2018 Jul 10;13:4015-4027. doi: 10.2147/IJN.S164393. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30022824 Free PMC article.
-
Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity.J Control Release. 2019 Apr 10;299:31-43. doi: 10.1016/j.jconrel.2019.02.030. Epub 2019 Feb 21. J Control Release. 2019. PMID: 30797868 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

