Investigating BB0405 as a novel Borrelia afzelii vaccination candidate in Lyme borreliosis
- PMID: 33637813
- PMCID: PMC7910573
- DOI: 10.1038/s41598-021-84130-y
Investigating BB0405 as a novel Borrelia afzelii vaccination candidate in Lyme borreliosis
Abstract
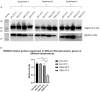
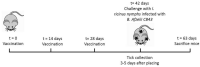
BB0405 is a surface exposed Borrelia burgdorferi protein and its vaccination protected mice against B. burgdorferi infection. As BB0405 is highly conserved across different B. burgdorferi sensu lato species, we investigated whether vaccination with recombinant BB0405 or through intradermal bb0405 DNA tattoo vaccination could provide protection against different Borrelia species, specifically against Borrelia afzelii, the predominant B. burgdorferi sensu lato genospecies causing Lyme borreliosis across Eurasia. We immunized C3H/HeN mice with recombinant BB0405 or with a codon-optimized bb0405 DNA vaccine using the pVAC plasmid and immunized corresponding control groups mice with only adjuvant or empty vectors. We subsequently subjected these immunized mice to a tick challenge with B. afzelii CB43-infected Ixodes ricinus nymphs. Upon vaccination, recombinant BB0405 induced a high total IgG response, but bb0405 DNA vaccination did not elicit antibody responses. Both vaccine formulations did not provide protection against Borrelia afzelii strain CB43 after tick challenge. In an attempt to understand the lack of protection of the recombinant vaccine, we determined expression of BB0405 and showed that B. afzelii CB43 spirochetes significantly and drastically downregulate the expression of BB0405 protein at 37 °C compared to 33 °C, where as in B. burgdorferi B31 spirochetes expression levels remain unaltered. Vaccination with recombinant BB0405 was previously shown to protect against B. burgdorferi sensu stricto. Here we show that vaccination with either recombinant BB0405 (or non-immunogenic bb0405 DNA), despite being highly conserved among B. burgdorferi sl genospecies, does not provide cross-protection against B. afzelii, mostly likely due to downregulation of this protein in B. afzelii in the mammalian host.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Tick-Tattoo: DNA Vaccination Against B. burgdorferi or Ixodes scapularis Tick Proteins.Front Immunol. 2021 Feb 25;12:615011. doi: 10.3389/fimmu.2021.615011. eCollection 2021. Front Immunol. 2021. PMID: 33717102 Free PMC article.
-
Rapid outer-surface protein C DNA tattoo vaccination protects against Borrelia afzelii infection.Gene Ther. 2014 Dec;21(12):1051-7. doi: 10.1038/gt.2014.87. Epub 2014 Oct 2. Gene Ther. 2014. PMID: 25273355
-
Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody.J Immunol. 2001 Jun 15;166(12):7398-403. doi: 10.4049/jimmunol.166.12.7398. J Immunol. 2001. PMID: 11390491
-
Lyme borreliosis vaccination: the facts, the challenge, the future.Trends Parasitol. 2011 Jan;27(1):40-7. doi: 10.1016/j.pt.2010.06.006. Epub 2010 Jun 30. Trends Parasitol. 2011. PMID: 20594913 Review.
-
Lyme disease and current aspects of immunization.Arthritis Res. 2002;4(1):20-9. doi: 10.1186/ar379. Epub 2001 Sep 28. Arthritis Res. 2002. PMID: 11879534 Free PMC article. Review.
Cited by
-
Past, present, and future of Lyme disease vaccines: antigen engineering approaches and mechanistic insights.Expert Rev Vaccines. 2022 Oct;21(10):1405-1417. doi: 10.1080/14760584.2022.2102484. Epub 2022 Jul 22. Expert Rev Vaccines. 2022. PMID: 35836340 Free PMC article. Review.
-
Protective efficacy of mutant strains of Borrelia burgdorferi as potential reservoir host-targeted biologics against Lyme disease.bioRxiv [Preprint]. 2025 Jul 9:2025.07.09.663885. doi: 10.1101/2025.07.09.663885. bioRxiv. 2025. PMID: 40672219 Free PMC article. Preprint.
-
Pathogenicity and virulence of Borrelia burgdorferi.Virulence. 2023 Dec;14(1):2265015. doi: 10.1080/21505594.2023.2265015. Epub 2023 Oct 9. Virulence. 2023. PMID: 37814488 Free PMC article. Review.
-
Probing an Ixodes ricinus salivary gland yeast surface display with tick-exposed human sera to identify novel candidates for an anti-tick vaccine.Sci Rep. 2021 Aug 3;11(1):15745. doi: 10.1038/s41598-021-92538-9. Sci Rep. 2021. PMID: 34344917 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

