LRP1 mediates the IGF-1-induced GLUT1 expression on the cell surface and glucose uptake in Müller glial cells
- PMID: 33637845
- PMCID: PMC7910306
- DOI: 10.1038/s41598-021-84090-3
LRP1 mediates the IGF-1-induced GLUT1 expression on the cell surface and glucose uptake in Müller glial cells
Abstract
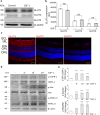
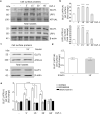
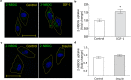
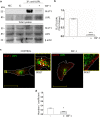
Insulin-like Growth Factor-1 (IGF-1) is involved in the normal development and survival of retinal cells. Low-density lipoprotein Receptor-related Protein-1 (LRP1) plays a key role on the regulation of several membrane proteins, such as the IGF-1 receptor (IGF-1R). In brain astrocytes, LRP1 interact with IGF-1R and the glucose transporter type 1 (GLUT1), regulating the glucose uptake in these cells. Although GLUT1 is expressed in retinal Müller Glial Cells (MGCs), its regulation is not clear yet. Here, we investigated whether IGF-1 modulates GLUT1 traffic to plasma membrane (PM) and glucose uptake, as well as the involvement of LRP1 in this process in the human Müller glial-derived cell line (MIO-M1). We found that IGF-1 produced GLUT1 translocation to the PM, in a time-dependent manner involving the intracellular signaling activation of MAPK/ERK and PI3K/Akt pathways, and generated a significant glucose uptake. Moreover, we found a molecular association between LRP1 and GLUT1, which was significantly reduced by IGF-1. Finally, cells treated with specific siRNA for LRP1 showed an impaired GLUT1 expression on PM and decreased glucose uptake induced by IGF-1. We conclude that IGF-1 regulates glucose homeostasis in MGCs involving the expression of LRP1.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bringmann A, et al. Human Muller glial cells: altered potassium channel activity in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1999;40:3316–3323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

