The cancer glycocalyx mediates intravascular adhesion and extravasation during metastatic dissemination
- PMID: 33637851
- PMCID: PMC7910477
- DOI: 10.1038/s42003-021-01774-2
The cancer glycocalyx mediates intravascular adhesion and extravasation during metastatic dissemination
Abstract
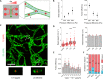
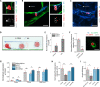
The glycocalyx on tumor cells has been recently identified as an important driver for cancer progression, possibly providing critical opportunities for treatment. Metastasis, in particular, is often the limiting step in the survival to cancer, yet our understanding of how tumor cells escape the vascular system to initiate metastatic sites remains limited. Using an in vitro model of the human microvasculature, we assess here the importance of the tumor and vascular glycocalyces during tumor cell extravasation. Through selective manipulation of individual components of the glycocalyx, we reveal a mechanism whereby tumor cells prepare an adhesive vascular niche by depositing components of the glycocalyx along the endothelium. Accumulated hyaluronic acid shed by tumor cells subsequently mediates adhesion to the endothelium via the glycoprotein CD44. Trans-endothelial migration and invasion into the stroma occurs through binding of the isoform CD44v to components of the sub-endothelial extra-cellular matrix. Targeting of the hyaluronic acid-CD44 glycocalyx complex results in significant reduction in the extravasation of tumor cells. These studies provide evidence of tumor cells repurposing the glycocalyx to promote adhesive interactions leading to cancer progression. Such glycocalyx-mediated mechanisms may be therapeutically targeted to hinder metastasis and improve patient survival.
Conflict of interest statement
The authors declare the following competing interests: R.D.K. is a co‐founder of AIM Biotech that markets microfluidic systems for 3D culture. Funding support is also provided by Amgen, Biogen, and Gore. The remaining authors declare no competing interests.
Figures



Similar articles
-
Dynamic interplay between breast cancer cells and normal endothelium mediates the expression of matrix macromolecules, proteasome activity and functional properties of endothelial cells.Biochim Biophys Acta. 2014 Aug;1840(8):2549-59. doi: 10.1016/j.bbagen.2014.02.019. Epub 2014 Feb 26. Biochim Biophys Acta. 2014. PMID: 24582970
-
Cancer cell glycocalyx mediates mechanotransduction and flow-regulated invasion.Integr Biol (Camb). 2013 Nov;5(11):1334-43. doi: 10.1039/c3ib40057c. Epub 2013 Sep 30. Integr Biol (Camb). 2013. PMID: 24077103 Free PMC article.
-
An integrated assay to probe endothelial glycocalyx-blood cell interactions under flow in mechanically and biochemically well-defined environments.Matrix Biol. 2019 May;78-79:47-59. doi: 10.1016/j.matbio.2018.12.002. Epub 2019 Jan 8. Matrix Biol. 2019. PMID: 30633963
-
Mechanisms of triple-negative breast cancer extravasation: Impact of the physical environment and endothelial glycocalyx.FASEB J. 2024 Jul 15;38(13):e23785. doi: 10.1096/fj.202400380R. FASEB J. 2024. PMID: 38949120 Review.
-
Hyaluronan-CD44 interactions as potential targets for cancer therapy.FEBS J. 2011 May;278(9):1429-43. doi: 10.1111/j.1742-4658.2011.08071.x. Epub 2011 Mar 25. FEBS J. 2011. PMID: 21362138 Free PMC article. Review.
Cited by
-
Convergent Approaches to Delineate the Metabolic Regulation of Tumor Invasion by Hyaluronic Acid Biosynthesis.Adv Healthc Mater. 2023 Jun;12(14):e2202224. doi: 10.1002/adhm.202202224. Epub 2022 Dec 21. Adv Healthc Mater. 2023. PMID: 36479976 Free PMC article.
-
Hierarchical Vessel Network-Supported Tumor Model-on-a-Chip Constructed by Induced Spontaneous Anastomosis.ACS Appl Mater Interfaces. 2023 Feb 8;15(5):6431-6441. doi: 10.1021/acsami.2c19453. Epub 2023 Jan 24. ACS Appl Mater Interfaces. 2023. PMID: 36693007 Free PMC article.
-
Scanning Probe Microscopy Techniques for Studying the Cell Glycocalyx.Cells. 2023 Dec 6;12(24):2778. doi: 10.3390/cells12242778. Cells. 2023. PMID: 38132098 Free PMC article. Review.
-
Mechanoregulation of Vascular Endothelial Growth Factor Receptor 2 in Angiogenesis.Front Cardiovasc Med. 2022 Jan 11;8:804934. doi: 10.3389/fcvm.2021.804934. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35087885 Free PMC article. Review.
-
Glycomimetics for the inhibition and modulation of lectins.Chem Soc Rev. 2023 Jun 6;52(11):3663-3740. doi: 10.1039/d2cs00954d. Chem Soc Rev. 2023. PMID: 37232696 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

