Facile synthesis and antimycobacterial activity of isoniazid, pyrazinamide and ciprofloxacin derivatives
- PMID: 33638304
- PMCID: PMC8113106
- DOI: 10.1111/cbdd.13836
Facile synthesis and antimycobacterial activity of isoniazid, pyrazinamide and ciprofloxacin derivatives
Abstract
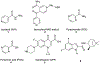
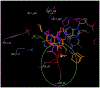
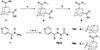
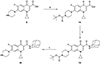
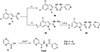
Several rationally designed isoniazid (INH), pyrazinamide (PZA) and ciprofloxacin (CPF) derivatives were conveniently synthesized and evaluated in vitro against H37Rv Mycobacterium tuberculosis (M. tb) strain. CPF derivative 16 displayed a modest activity (MIC = 16 µg/ml) and was docked into the M. tb DNA gyrase. Isoniazid-pyrazinoic acid (INH-POA) hybrid 21a showed the highest potency in our study (MIC = 2 µg/ml). It also retained its high activity against the other tested M. tb drug-sensitive strain (DS) V4207 (MIC = 4 µg/ml) and demonstrated negligible cytotoxicity against Vero cells (IC50 ≥ 64 µg/ml). Four tested drug-resistant (DR) M. tb strains were refractory to 21a, similar to INH, whilst being sensitive to CPF. Compound 21a was also inactive against two non-tuberculous mycobacterial (NTM) strains, suggesting its selective activity against M. tb. The noteworthy activity of 21a against DS strains and its low cytotoxicity highlight its potential to treat DS M. tb.
Keywords: ciprofloxacin; hybrid molecules; indoleamides; isoniazid; pyrazinamide; tuberculosis.
© 2021 John Wiley & Sons Ltd.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

