Diagnostic value of amyloid-PET and tau-PET: a head-to-head comparison
- PMID: 33638661
- PMCID: PMC8175315
- DOI: 10.1007/s00259-021-05246-x
Diagnostic value of amyloid-PET and tau-PET: a head-to-head comparison
Abstract
Purpose: Assess the individual and combined diagnostic value of amyloid-PET and tau-PET in a memory clinic population.
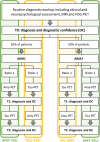
Methods: Clinical reports of 136 patients were randomly assigned to two diagnostic pathways: AMY-TAU, amyloid-PET is presented before tau-PET; and TAU-AMY, tau-PET is presented before amyloid-PET. Two neurologists independently assessed all reports with a balanced randomized design, and expressed etiological diagnosis and diagnostic confidence (50-100%) three times: (i) at baseline based on the routine diagnostic workup, (ii) after the first exam (amyloid-PET for the AMY-TAU pathway, and tau-PET for the TAU-AMY pathway), and (iii) after the remaining exam. The main outcomes were changes in diagnosis (from AD to non-AD or vice versa) and in diagnostic confidence.
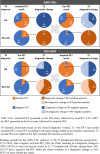
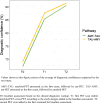
Results: Amyloid-PET and tau-PET, when presented as the first exam, resulted in a change of etiological diagnosis in 28% (p = 0.006) and 28% (p < 0.001) of cases, and diagnostic confidence increased by 18% (p < 0.001) and 19% (p < 0.001) respectively, with no differences between exams (p > 0.05). We observed a stronger impact of a negative amyloid-PET versus a negative tau-PET (p = 0.014). When added as the second exam, amyloid-PET and tau-PET resulted in a further change in etiological diagnosis in 6% (p = 0.077) and 9% (p = 0.149) of cases, and diagnostic confidence increased by 4% (p < 0.001) and 5% (p < 0.001) respectively, with no differences between exams (p > 0.05).
Conclusion: Amyloid-PET and tau-PET significantly impacted diagnosis and diagnostic confidence in a similar way, although a negative amyloid-PET has a stronger impact on diagnosis than a negative tau-PET. Adding either of the two as second exam further improved diagnostic confidence.
Trial number: PB 2016-01346.
Keywords: Amyloid; Florbetapir; Flortaucipir; Flutemetamol; PET; Tau.
Conflict of interest statement
Daniele Altomare declares that he has no conflict of interest.
Camilla Caprioglio declares that she has no conflict of interest.
Frédéric Assal declares that he has no conflict of interest.
Gilles Allali declares that he has no conflict of interest.
Aline Mendes declares that she has no conflict of interest.
Federica Ribaldi declares that she has no conflict of interest.
Kelly Ceyzeriat declares that she has no conflict of interest.
Marta Martins declares that she has no conflict of interest.
Szymon Tomczyk declares that he has no conflict of interest.
Sara Stampacchia declares that she has no conflict of interest.
Alessandra Dodich declares that she has no conflict of interest.
Marina Boccardi declares that she has no conflict of interest.
Christian Chicherio declares that he has no conflict of interest.
Giovanni B. Frisoni reports grants from Alzheimer Forum Suisse, Académie Suisse des Sciences Médicales, Avid Radiopharmaceuticals, Biogen, GE International, Guerbert, Association Suisse pour la Recherche sur l’Alzheimer, IXICO, Merz Pharma, Nestlé, Novartis, Piramal, Roche, Siemens, Teva Pharmaceutical Industries, Vifor Pharma, and Alzheimer’s Association; he has received personal fees from AstraZeneca, Avid Radiopharmaceuticals, Elan Pharmaceuticals, GE International, Lundbeck, Pfizer, and TauRx Therapeutics.
Valentina Garibotto received financial support for research through her institution from Siemens Healthineers, GE Healthcare, Life Molecular Imaging, Cerveau Technologies, Roche, Merck.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical