A follow-up study of a randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-month depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer
- PMID: 33638810
- PMCID: PMC8064970
- DOI: 10.1007/s12282-020-01205-w
A follow-up study of a randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-month depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer
Abstract
Background: Previously, we conducted the 5-year open-label, randomized controlled trial (RCT) of leuprorelin adjuvant therapy in post-operative premenopausal patients with endocrine-responsive breast cancer, which was a pilot study to investigate the optimal duration of leuprorelin treatment. Since, however, long-term outcomes became required for the adjuvant endocrine therapy, we performed this follow-up observation study.
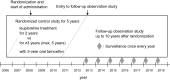
Methods: Follow-up observation study was performed up to 10th year after randomization, continuing RCT to evaluate the efficacy and safety of leuprorelin every 3 months for ≥ 3 versus 2 years, with daily tamoxifen for 5 years. Primary endpoints were disease-free survival (DFS) and 2-year landmark DFS.
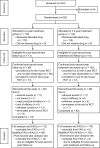
Results: Eligible patients (N = 222) were randomly assigned to receive leuprorelin for either 2 years (N = 112) or ≥ 3 years (N = 110) with tamoxifen. Leuprorelin treatment for ≥ 3 years versus 2 years provided no significant difference in DFS (HR 0.944, 95% CI 0.486-1.8392) or 2-year landmark DFS (N = 99 and 102 in 2-year and ≥ 3-year groups, HR 0.834, 0.397-1.753). In small, higher-risk subgroup (n = 17); however, 2-year landmark DFS in ≥ 3-year group was significantly longer (HR 0.095, 0.011-0.850) than that in 2-year group. The incidence of bone-related adverse events was around 5% in both groups.
Conclusions: Adjuvant leuprorelin treatment for ≥ 3 years with tamoxifen only showed similar efficacy and safety profiles to those for 2 years in analyses among all patients but suggested greater benefit in higher-risk patients. No new safety signal was identified for long-term leuprorelin treatment.
Trial registration number: Not applicable. This was an observational study.
Keywords: Adjuvant endocrine therapy; Endocrine-responsive breast cancer; LH-RH agonist; Ovarian function suppression; Premenopausal patient.
Conflict of interest statement
Dr. Kurebayashi received grants and personal fees from Takeda Pharmaceutical Company Limited, Eisai Co., Ltd., and Chugai Pharmaceutical Co., Ltd., outside the submitted work. Dr. Toyama received personal fees from AstraZeneca K.K., outside the submitted work. Dr. Toyama also received grants and personal fees from Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo Company, Limited, Eli Lilly Japan K.K., Novartis Pharma K.K., Pfizer Japan Inc., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Nippon Kayaku Co., Ltd., and Kyowa Kirin Co., Ltd., outside the submitted work. Prof. Ohashi received grants from Medical Member System, outside the submitted work. Prof. Ohashi also received personal fees from Statcom Company Limited, Daiichi Sankyo Company, Limited, Chugai Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Taiho Pharmaceutical Co., Ltd., Sanofi K.K., and EP-CRSU Co., Ltd., outside the submitted work. The other authors declare that they have no competing interests.
Figures



Similar articles
-
A randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-months depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer.Breast Cancer. 2016 May;23(3):499-509. doi: 10.1007/s12282-015-0593-z. Epub 2015 Feb 6. Breast Cancer. 2016. PMID: 25655898 Free PMC article. Clinical Trial.
-
Comparison of quality of life between 2-year and 3-or-more-year administration of leuprorelin acetate every-3-months depot in combination with tamoxifen as adjuvant endocrine treatment in premenopausal patients with endocrine-responsive breast cancer: a randomized controlled trial.Support Care Cancer. 2018 Mar;26(3):933-945. doi: 10.1007/s00520-017-3914-2. Epub 2017 Oct 24. Support Care Cancer. 2018. PMID: 29063390 Free PMC article. Clinical Trial.
-
Efficacy and safety of leuprorelin acetate 6-month depot, TAP-144-SR (6M), in combination with tamoxifen in postoperative, premenopausal patients with hormone receptor-positive breast cancer: a phase III, randomized, open-label, parallel-group comparative study.Breast Cancer. 2017 Jan;24(1):161-170. doi: 10.1007/s12282-016-0691-6. Epub 2016 Mar 26. Breast Cancer. 2017. PMID: 27017207 Free PMC article. Clinical Trial.
-
Luteininzing hormone releasing hormones analogs in combination with tamoxifen for the adjuvant treatment of premenopausal women with hormone receptor positive breast cancer.Expert Opin Pharmacother. 2017 Sep;18(13):1357-1362. doi: 10.1080/14656566.2017.1363181. Epub 2017 Aug 10. Expert Opin Pharmacother. 2017. PMID: 28764603 Review.
-
Adverse effects of tamoxifen treatment on bone mineral density in premenopausal patients with breast cancer: a systematic review and meta-analysis.Breast Cancer. 2024 Jul;31(4):717-725. doi: 10.1007/s12282-024-01586-2. Epub 2024 Apr 27. Breast Cancer. 2024. PMID: 38671211
Cited by
-
Targeting sex steroid biosynthesis for breast and prostate cancer therapy.Nat Rev Cancer. 2023 Oct;23(10):686-709. doi: 10.1038/s41568-023-00609-y. Epub 2023 Sep 8. Nat Rev Cancer. 2023. PMID: 37684402 Review. No abstract available.
-
An Overview of Long-Acting GnRH Agonists in Premenopausal Breast Cancer Patients: Survivorship Challenges and Management.Curr Oncol. 2024 Jul 25;31(8):4209-4224. doi: 10.3390/curroncol31080314. Curr Oncol. 2024. PMID: 39195297 Free PMC article. Review.
-
Bone Effects of Anti-Cancer Treatments in 2024.Calcif Tissue Int. 2025 Mar 27;116(1):54. doi: 10.1007/s00223-025-01362-0. Calcif Tissue Int. 2025. PMID: 40146323 Free PMC article. Review.
References
-
- Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

