Mitochondrial fission is a critical modulator of mutant APP-induced neural toxicity
- PMID: 33639169
- PMCID: PMC8042169
- DOI: 10.1016/j.jbc.2021.100469
Mitochondrial fission is a critical modulator of mutant APP-induced neural toxicity
Abstract
Alterations in mitochondrial fission may contribute to the pathophysiology of several neurodegenerative diseases, including Alzheimer's disease (AD). However, we understand very little about the normal functions of fission or how fission disruption may interact with AD-associated proteins to modulate pathogenesis. Here we show that loss of the central mitochondrial fission protein dynamin-related protein 1 (Drp1) in CA1 and other forebrain neurons markedly worsens the learning and memory of mice expressing mutant human amyloid precursor protein (hAPP) in neurons. In cultured neurons, Drp1KO and hAPP converge to produce mitochondrial Ca2+ (mitoCa2+) overload, despite decreasing mitochondria-associated ER membranes (MAMs) and cytosolic Ca2+. This mitoCa2+ overload occurs independently of ATP levels. These findings reveal a potential mechanism by which mitochondrial fission protects against hAPP-driven pathology.
Keywords: Alzheimer’s disease; Drp1; amyloid precursor protein (APP); mitochondria; mitochondrial calcium; mitochondrial fission; neurodegeneration; neurodegenerative disease.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

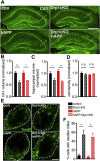

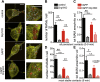
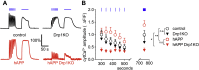
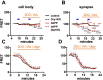
Similar articles
-
Hippocampal mutant APP and amyloid beta-induced cognitive decline, dendritic spine loss, defective autophagy, mitophagy and mitochondrial abnormalities in a mouse model of Alzheimer's disease.Hum Mol Genet. 2018 Apr 15;27(8):1332-1342. doi: 10.1093/hmg/ddy042. Hum Mol Genet. 2018. PMID: 29408999 Free PMC article.
-
Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease.Hum Mol Genet. 2016 Nov 15;25(22):4881-4897. doi: 10.1093/hmg/ddw312. Hum Mol Genet. 2016. PMID: 28173111 Free PMC article.
-
Dynamin-related protein 1 is required for normal mitochondrial bioenergetic and synaptic function in CA1 hippocampal neurons.Cell Death Dis. 2015 Apr 16;6(4):e1725. doi: 10.1038/cddis.2015.94. Cell Death Dis. 2015. PMID: 25880092 Free PMC article.
-
Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Mitochondrial Dysfunction for Alzheimer's Disease.Mol Neurobiol. 2022 Jan;59(1):535-555. doi: 10.1007/s12035-021-02612-6. Epub 2021 Nov 2. Mol Neurobiol. 2022. PMID: 34725778 Review.
-
Mechanistic and therapeutic role of Drp1 in the pathogenesis of Alzheimer's disease.Eur J Neurosci. 2022 Nov;56(9):5516-5531. doi: 10.1111/ejn.15611. Epub 2022 Feb 12. Eur J Neurosci. 2022. PMID: 35078269 Review.
Cited by
-
Mitochondria in health, disease, and aging.Physiol Rev. 2023 Oct 1;103(4):2349-2422. doi: 10.1152/physrev.00058.2021. Epub 2023 Apr 6. Physiol Rev. 2023. PMID: 37021870 Free PMC article. Review.
-
Mitochondrial quality control disorder in neurodegenerative disorders: Potential and advantages of traditional Chinese medicines.J Pharm Anal. 2025 Apr;15(4):101146. doi: 10.1016/j.jpha.2024.101146. Epub 2024 Nov 14. J Pharm Anal. 2025. PMID: 40291018 Free PMC article. Review.
-
Presenilins and mitochondria-an intriguing link: mini-review.Front Neurosci. 2023 Jul 28;17:1249815. doi: 10.3389/fnins.2023.1249815. eCollection 2023. Front Neurosci. 2023. PMID: 37575294 Free PMC article. Review.
-
Reciprocal regulation of oxidative stress and mitochondrial fission augments parvalbumin downregulation through CDK5-DRP1- and GPx1-NF-κB signaling pathways.Cell Death Dis. 2024 Sep 30;15(9):707. doi: 10.1038/s41419-024-07050-5. Cell Death Dis. 2024. PMID: 39349423 Free PMC article.
-
Amyloid β-based therapy for Alzheimer's disease: challenges, successes and future.Signal Transduct Target Ther. 2023 Jun 30;8(1):248. doi: 10.1038/s41392-023-01484-7. Signal Transduct Target Ther. 2023. PMID: 37386015 Free PMC article. Review.
References
-
- Nakamura K., Nemani V.M., Azarbal F., Skibinski G., Levy J.M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., Sesaki H., Cheng Y., Finkbeiner S., Nussbaum R.L., Masliah E. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 2011;286:20710–20726. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

