Searching for host immune-microbiome mechanisms in obsessive-compulsive disorder: A narrative literature review and future directions
- PMID: 33639178
- PMCID: PMC8106658
- DOI: 10.1016/j.neubiorev.2021.02.034
Searching for host immune-microbiome mechanisms in obsessive-compulsive disorder: A narrative literature review and future directions
Abstract
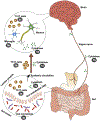
Obsessive-compulsive disorder (OCD) is disabling and often treatment-refractory. Host immunity and gut microbiota have bidirectional communication with each other and with the brain. Perturbations to this axis have been implicated in neuropsychiatric disorders, but immune-microbiome signaling in OCD is relatively underexplored. We review support for further pursuing such investigations in OCD, including: 1) gut microbiota has been associated with OCD, but causal pathogenic mechanisms remain unclear; 2) early environmental risk factors for OCD overlap with critical periods of immune-microbiome development; 3) OCD is associated with increased risk of immune-mediated disorders and changes in immune parameters, which are separately associated with the microbiome; and 4) gut microbiome manipulations in animal models are associated with changes in immunity and some obsessive-compulsive symptoms. Theoretical pathogenic mechanisms could include microbiota programming of cytokine production, promotion of expansion and trafficking of peripheral immune cells to the CNS, and regulation of microglial function. Immune-microbiome signaling in OCD requires further exploration, and may offer novel insights into pathogenic mechanisms and potential treatment targets for this disabling disorder.
Keywords: Gut microbiome; Gut-brain-immune axis; Immune; Inflammation; Microbiota; Neuroinflammation; Obsessive-compulsive behavior; Obsessive-compulsive disorder.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Financial Disclosures
Drs. Troyer, Kohn, Ecklu-Mensah, Aleti, Rosenberg and Hong have no commercial financial conflicts of interest to disclose.
Figures
Similar articles
-
Human microbiota from drug-naive patients with obsessive-compulsive disorder drives behavioral symptoms and neuroinflammation via succinic acid in mice.Mol Psychiatry. 2024 Jun;29(6):1782-1797. doi: 10.1038/s41380-024-02424-9. Epub 2024 Jan 25. Mol Psychiatry. 2024. PMID: 38273106
-
Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder.Sci Rep. 2022 Jan 27;12(1):1448. doi: 10.1038/s41598-022-05480-9. Sci Rep. 2022. PMID: 35087123 Free PMC article.
-
Microbial Reprogramming in Obsessive-Compulsive Disorders: A Review of Gut-Brain Communication and Emerging Evidence.Int J Mol Sci. 2023 Jul 26;24(15):11978. doi: 10.3390/ijms241511978. Int J Mol Sci. 2023. PMID: 37569349 Free PMC article. Review.
-
"WHAT'S BUGGING THE GUT IN OCD?" A REVIEW OF THE GUT MICROBIOME IN OBSESSIVE-COMPULSIVE DISORDER.Depress Anxiety. 2016 Mar;33(3):171-8. doi: 10.1002/da.22454. Epub 2015 Dec 2. Depress Anxiety. 2016. PMID: 26629974 Review.
-
Implication of microbiota gut-brain axis in the manifestation of obsessive-compulsive disorder: Preclinical and clinical evidence.Eur J Pharmacol. 2023 Oct 15;957:176014. doi: 10.1016/j.ejphar.2023.176014. Epub 2023 Aug 22. Eur J Pharmacol. 2023. PMID: 37619786 Review.
Cited by
-
Human microbiota from drug-naive patients with obsessive-compulsive disorder drives behavioral symptoms and neuroinflammation via succinic acid in mice.Mol Psychiatry. 2024 Jun;29(6):1782-1797. doi: 10.1038/s41380-024-02424-9. Epub 2024 Jan 25. Mol Psychiatry. 2024. PMID: 38273106
-
The Gut-Brain Axis and the Microbiome in Anxiety Disorders, Post-Traumatic Stress Disorder and Obsessive-Compulsive Disorder.Curr Neuropharmacol. 2024;22(5):866-883. doi: 10.2174/1570159X21666230222092029. Curr Neuropharmacol. 2024. PMID: 36815632 Free PMC article. Review.
-
Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder.Sci Rep. 2022 Jan 27;12(1):1448. doi: 10.1038/s41598-022-05480-9. Sci Rep. 2022. PMID: 35087123 Free PMC article.
-
Microbial Reprogramming in Obsessive-Compulsive Disorders: A Review of Gut-Brain Communication and Emerging Evidence.Int J Mol Sci. 2023 Jul 26;24(15):11978. doi: 10.3390/ijms241511978. Int J Mol Sci. 2023. PMID: 37569349 Free PMC article. Review.
References
-
- Aden K, Rehman A, Waschina S, Pan WH, Walker A, Lucio M, Nunez AM, Bharti R, Zimmerman J, Bethge J, Schulte B, Schulte D, Franke A, Nikolaus S, Schroeder JO, Vandeputte D, Raes J, Szymczak S, Waetzig GH, Zeuner R, Schmitt-Kopplin P, Kaleta C, Schreiber S, Rosenstiel P, 2019. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 157, 1279–1292.e11. 10.1053/j.gastro.2019.07.025 - DOI - PubMed
-
- APA, 2013. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth. ed. American Psychiatric Association, Washington, D.C.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

