Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection
- PMID: 33639492
- PMCID: PMC7897407
- DOI: 10.1016/j.jcv.2021.104781
Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection
Abstract
Objectives: Antigen rapid diagnostic tests (Ag-RDT) have been developed as reliable tools to control the SARS-CoV-2 pandemic. The objective of our study was to evaluate the diagnostic performance of two Ag-RDTs.
Methods: We evaluated CerTest SARS-CoV-2 Ag One Step Card Test and Panbio COVID-19 Ag Rapid Test Device Ag-RDTs. We included 320 nasopharyngeal samples: 150 PCR negative samples to assess the specificity and 170 PCR positive samples to evaluate the sensitivity. We also evaluated their sensitivity according to cycle threshold (Ct) values and the time from the onset of symptoms. Tests were compared using the McNemar's test and agreement was evaluated using the kappa score (k).
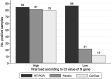
Results: Both Ag-RDTs showed a specificity of 100 %. Overall sensitivity was 53.5 % for CerTest and 60.0 % for Panbio. For samples with Ct≤ 25, sensitivity was 94.0 % for CerTest and 96.4 % for Panbio (p = 0.500). Regarding samples with Ct>25, sensitivity was 14.0 % for CerTest and 24.4 % for Panbio (p = 0.004). Sensitivity for samples within the first 5 days after the onset of symptoms were 84.8 % for CerTest and 91.3 % for Panbio (p = 0.250) and notably decreased for samples taken after the fifth day. Both Ag-RDTs showed an excellent agreement between them (agreement = 96.7 %, k = 0.920). Agreement with PCR was also excellent for high viral load samples (Ct<25) for CerTest (98.0 %, k = 0.954) and Panbio (98.8 %, k = 0.973).
Conclusions: CerTest SARS-CoV-2 and Panbio COVID-19 Ag showed excellent performance and agreement results for samples with high viral loads (Ct ≤ 25) or samples taken within the first 5 days after the onset of symptoms.
Keywords: Antigen rapid diagnostic test; COVID-19; CerTest SARS-CoV-2 Ag; Lateral flow immunoassay; Panbio COVID-19 Ag; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
References
-
- World Health Organization, Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update, (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... (Accessed 1 October 2021).
-
- World Health Organization, Advice on the use of point-of-care immunodiagnostic tests for COVID-19, (n.d.). https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-p.... (Accessed 19 August 2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous