Multiple endocrinopathies, hypercalcaemia and pancreatitis following combined immune checkpoint inhibitor use- case report and review of literature
- PMID: 33639911
- PMCID: PMC7912868
- DOI: 10.1186/s12902-021-00693-x
Multiple endocrinopathies, hypercalcaemia and pancreatitis following combined immune checkpoint inhibitor use- case report and review of literature
Abstract
Background: Immune checkpoint inhibitors (ICIs) are a novel class of oncological agents which are used to treat a number of malignancies. To date seven agents have been approved by the Food and Drug Administration (FDA) to treat both solid and haematological malignancies. Despite their efficacy they have been associated with a number of endocrinopathies. We report a unique case of hypophysitis, thyroiditis, severe hypercalcaemia and pancreatitis following combined ICI therapy.
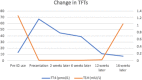
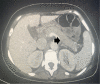
Case presentation: A 46-year old Caucasian female with a background history of malignant melanoma and lung metastases presented to the emergency department with lethargy, nausea, palpitations and tremors. She had been started on a combination of nivolumab and ipilimumab 24 weeks earlier. Initial investigations revealed thyrotoxicosis with a thyroid stimulating hormone (TSH) of < 0.01 (0.38-5.33) mIU/L, free T4 of 66.9 (7-16) pmol/.L. TSH receptor and thyroperoxidase antibodies were negative. She was diagnosed with thyroiditis and treated with a beta blocker. Six weeks later she represented with polyuria and polydipsia. A corrected calcium of 3.54 (2.2-2.5) mmol/l and parathyroid hormone (PTH) of 9 (10-65) pg/ml confirmed a diagnosis of non-PTH mediated hypercalcaemia. PTH-related peptide and 1, 25-dihydroxycholecalciferol levels were within the normal range. Cross-sectional imaging and a bone scan out ruled bone metastases but did reveal an incidental finding of acute pancreatitis - both glucose and amylase levels were normal. The patient was treated with intravenous hydration and zoledronic acid. Assessment of the hypothalamic-pituitary-adrenal (HPA) axis uncovered adrenocorticotrophic hormone (ACTH) deficiency with a morning cortisol of 17 nmol/L. A pituitary Magnetic Resonance Image (MRI) was unremarkable. Given her excellent response to ICI therapy she remained on ipilimumab and nivolumab. On follow-up this patient's thyrotoxicosis had resolved without anti-thyroid mediations - consistent with a diagnosis of thyroiditis secondary to nivolumab use. Calcium levels normalised rapidly and remained normal. ACTH deficiency persisted, and she is maintained on oral prednisolone.
Conclusion: This is a remarkable case in which ACTH deficiency due to hypophysitis; thyroiditis; hypercalcaemia and pancreatitis developed in the same patient on ipilimumab and nivolumab combination therapy. We postulate that hypercalcaemia in this case was secondary to a combination of hyperthyroidism and secondary adrenal insufficiency.
Keywords: Case report; Hypercalcaemia; Hypophysitis; Immune checkpoint inhibitor; Thyroiditis.
Conflict of interest statement
Amar Agha, the senior author on this paper is also a member of the editorial review board of BMC Endocrine Disorders.
Figures
Similar articles
-
Adrenal Insufficiency and Thyrotoxicosis Following Combined Immune Checkpoint Inhibitor Use: A Case Report and Literature Review.Cureus. 2024 May 22;16(5):e60850. doi: 10.7759/cureus.60850. eCollection 2024 May. Cureus. 2024. PMID: 38910605 Free PMC article.
-
Recovery from secondary adrenal insufficiency in a patient with immune checkpoint inhibitor therapy induced hypophysitis.J Immunother Cancer. 2019 Sep 12;7(1):248. doi: 10.1186/s40425-019-0729-3. J Immunother Cancer. 2019. PMID: 31511065 Free PMC article.
-
Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: a case report and review of the literature.J Med Case Rep. 2019 Mar 26;13(1):88. doi: 10.1186/s13256-019-2002-2. J Med Case Rep. 2019. PMID: 30909965 Free PMC article. Review.
-
Fulminant ACTH decrease following diabetic ketoacidosis induced by immune checkpoint inhibitor combination therapy with nivolumab and ipilimumab: A case report.Medicine (Baltimore). 2023 Dec 22;102(51):e36664. doi: 10.1097/MD.0000000000036664. Medicine (Baltimore). 2023. PMID: 38134115 Free PMC article.
-
Clinical course and management of pembrolizumab-associated isolated adrenocorticotrophic hormone deficiency: a new case and literature review.Immunotherapy. 2021 Oct;13(14):1157-1163. doi: 10.2217/imt-2021-0061. Epub 2021 Aug 13. Immunotherapy. 2021. PMID: 34387129 Review.
Cited by
-
Adrenal Insufficiency and Thyrotoxicosis Following Combined Immune Checkpoint Inhibitor Use: A Case Report and Literature Review.Cureus. 2024 May 22;16(5):e60850. doi: 10.7759/cureus.60850. eCollection 2024 May. Cureus. 2024. PMID: 38910605 Free PMC article.
-
Hypercalcemia as an Immune-Related Adverse Event in a Patient Receiving Nivolumab and Ipilimumab for Metastatic Melanoma: A Case Report.Case Rep Oncol Med. 2025 Jul 10;2025:8600200. doi: 10.1155/crom/8600200. eCollection 2025. Case Rep Oncol Med. 2025. PMID: 40689241 Free PMC article.
-
Immune checkpoint inhibitors and acute kidney injury.Front Immunol. 2024 Feb 23;15:1353339. doi: 10.3389/fimmu.2024.1353339. eCollection 2024. Front Immunol. 2024. PMID: 38464524 Free PMC article. Review.
-
Immune Checkpoint Inhibitors in Cancer Treatment and Incidence of Pancreatitis.Cureus. 2024 Aug 28;16(8):e68043. doi: 10.7759/cureus.68043. eCollection 2024 Aug. Cureus. 2024. PMID: 39347217 Free PMC article. Review.
-
Thrombospondin-1, CD47, and SIRPα display cell-specific molecular signatures in human islets and pancreata.Am J Physiol Endocrinol Metab. 2023 Apr 1;324(4):E347-E357. doi: 10.1152/ajpendo.00221.2022. Epub 2023 Feb 15. Am J Physiol Endocrinol Metab. 2023. PMID: 36791324 Free PMC article.
References
-
- Ronan K, Othman EHS, McKenna S, Anderson C, Sheehan D, Griffin M, et al. Immunotherapy-induced endocrinopathies: a multicentre experience. J Clin Oncol. 2019;37:15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical