Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice
- PMID: 33639976
- PMCID: PMC7914043
- DOI: 10.1186/s12985-021-01515-1
Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice
Abstract
Background: Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 and broke out as a global pandemic in late 2019. The acidic pH environment of endosomes is believed to be essential for SARS-CoV-2 to be able to enter cells and begin replication. However, the clinical use of endosomal acidification inhibitors, typically chloroquine, has been controversial with this respect.
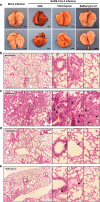
Methods: In this study, RT-qPCR method was used to detect the SARS-CoV-2N gene to evaluate viral replication. The CCK-8 assay was also used to evaluate the cytotoxic effect of SARS-CoV-2. In situ hybridization was used to examine the distribution of the SARS-CoV-2 gene in lung tissues. Hematoxylin and eosin staining was also used to evaluate virus-associated pathological changes in lung tissues.
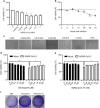
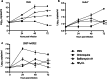
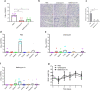
Results: In this study, analysis showed that endosomal acidification inhibitors, including chloroquine, bafilomycin A1 and NH4CL, significantly reduced the viral yields of SARS-CoV-2 in Vero E6, Huh-7 and 293T-ACE2 cells. Chloroquine and bafilomycin A1 also improved the viability and proliferation of Vero E6 cells after SARS-CoV-2 infection. Moreover, in the hACE2 transgenic mice model of SARS-CoV-2 infection, chloroquine and bafilomycin A1 reduced viral replication in lung tissues and alleviated viral pneumonia with reduced inflammatory exudation and infiltration in peribronchiolar and perivascular tissues, as well as improved structures of alveolar septum and pulmonary alveoli.
Conclusions: Our research investigated the antiviral effects of endosomal acidification inhibitors against SARS-CoV-2 in several infection models and provides an experimental basis for further mechanistic studies and drug development.
Keywords: Bafilomycin A1; Chloroquine; Endosomal acidification; SARS-CoV-2.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

