MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): a statistical analysis plan
- PMID: 33639999
- PMCID: PMC7913384
- DOI: 10.1186/s13063-021-05127-6
MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): a statistical analysis plan
Abstract
Background: Invasive mediastinal nodal staging is recommended by guidelines in selected patients with resectable non-small cell lung cancer (NSCLC). Endosonography is recommended as initial staging technique, followed by confirmatory mediastinoscopy in case of negative N2 or N3 cytology after endosonography. Confirmatory mediastinoscopy however is under debate owing its limited additional diagnostic value, its associated morbidity and its delay in the start of lung cancer treatment. The MEDIASTrial examines whether confirmatory mediastinoscopy can be safely omitted after negative endosonography in mediastinal nodal staging of NSCLC. The present work is the proposed statistical analysis plan of the clinical consequences of omitting mediastinoscopy, which is submitted before closure of the MEDIASTrial and before knowledge of any results was done to enhance transparency of scientific behaviour.
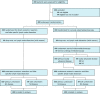
Methods: The primary outcome measure of this non-inferiority trial will be unforeseen N2 disease resulting from lobe-specific mediastinal lymph node dissection. For non-inferiority, the upper limit of the 95% confidence interval of the unforeseen N2 rate in the group without mediastinoscopy should not exceed 14.3% in order to probably have no negative impact on survival. Since this is a non-inferiority trial, both an intention to treat (ITT) and a per protocol (PP) analyses will be done. The ITT and the PP analyses should both indicate non-inferiority before the diagnostic strategy omitting mediastinoscopy will be interpreted as non-inferior to the strategy with mediastinoscopy. Secondary outcome measures include 30-day major morbidity and mortality, the total number of days of hospital care, overall and disease free 2-year survival, generic and disease-specific health related quality of life and cost-effectiveness and cost-utility of staging strategies with and without mediastinoscopy.
Discussion: The MEDIASTrial will determine if confirmatory mediastinoscopy can be omitted after tumour negative systematic endosonography in invasive mediastinal staging of patients with resectable NSCLC.
Trial registration: Netherlands Trial Register NL6344/NTR6528 . Registered on 2017 July 06.
Keywords: Endosonography; Mediastinal nodal staging; Mediastinoscopy; Non-small cell lung carcinoma; Statistical analysis plan; Thoracic surgery.
Conflict of interest statement
Dr. Bousema and Dr. van den Broek report grants from ZonMw and the Dutch Cancer Society, during the conduct of this study. Prof. Dr. Dijkgraaf and Prof. Dr. Verhagen have nothing to disclose. Dr. van der Heijden reports research grants from Pentax Medical, Philips Medical, unrelated to the content of this study. Consultancy for Philips-Volcano, Pentax Medical. Prof. Dr. Annema reports non-financial support from Hitachi Medical systems, non-financial support from Pentax, grants from Cook medical, outside the submitted work.
Figures

References
-
- Vilmann P, Clementsen PF, Colella S, Siemsen M, de Leyn P, Dumonceau J, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47(6):546–560. - PubMed
-
- Bousema JE, van Dorp M, Noyez VJJM, Dijkgraaf MGW, Annema JT, van den Broek FJC. Unforeseen N2 disease after negative endosonography findings with or without confirmatory mediastinoscopy in resectable non–small cell lung cancer: a systematic review and meta-analysis. J Thorac Oncol. 2019;14(6):979–992. doi: 10.1016/j.jtho.2019.02.032. - DOI - PubMed
-
- Bousema JE, Dijkgraaf MGW, Papen-Botterhuis NE, Schreurs HW, Maessen JG, van der Heijden EH, et al. MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): study protocol of a multicenter randomised controlled trial. BMC Surg. 2018;18(1):27. doi: 10.1186/s12893-018-0359-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

