Human hippocampal connectivity is stronger in olfaction than other sensory systems
- PMID: 33640412
- PMCID: PMC8096712
- DOI: 10.1016/j.pneurobio.2021.102027
Human hippocampal connectivity is stronger in olfaction than other sensory systems
Abstract
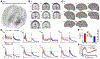
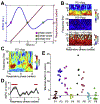
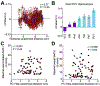
During mammalian evolution, primate neocortex expanded, shifting hippocampal functional networks away from primary sensory cortices, towards association cortices. Reflecting this rerouting, human resting hippocampal functional networks preferentially include higher association cortices, while those in rodents retained primary sensory cortices. Research on human visual, auditory and somatosensory systems shows evidence of this rerouting. Olfaction, however, is unique among sensory systems in its relative structural conservation throughout mammalian evolution, and it is unknown whether human primary olfactory cortex was subject to the same rerouting. We combined functional neuroimaging and intracranial electrophysiology to directly compare hippocampal functional networks across human sensory systems. We show that human primary olfactory cortex-including the anterior olfactory nucleus, olfactory tubercle and piriform cortex-has stronger functional connectivity with hippocampal networks at rest, compared to other sensory systems. This suggests that unlike other sensory systems, olfactory-hippocampal connectivity may have been retained in mammalian evolution. We further show that olfactory-hippocampal connectivity oscillates with nasal breathing. Our findings suggest olfaction might provide insight into how memory and cognition depend on hippocampal interactions.
Keywords: Functional connectivity; Hippocampal network; Olfactory system; fMRI; iEEG.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interests
The authors declare no conflict of interest.
Figures
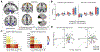

Similar articles
-
Long-Range Respiratory and Theta Oscillation Networks Depend on Spatial Sensory Context.J Neurosci. 2021 Dec 1;41(48):9957-9970. doi: 10.1523/JNEUROSCI.0719-21.2021. Epub 2021 Oct 19. J Neurosci. 2021. PMID: 34667070 Free PMC article.
-
The Organization of Mouse and Human Cortico-Hippocampal Networks Estimated by Intrinsic Functional Connectivity.Cereb Cortex. 2016 Dec;26(12):4497-4512. doi: 10.1093/cercor/bhw327. Epub 2016 Oct 25. Cereb Cortex. 2016. PMID: 27797832 Free PMC article.
-
Normal Olfactory Functional Connectivity Despite Lifelong Absence of Olfactory Experiences.Cereb Cortex. 2021 Jan 1;31(1):159-168. doi: 10.1093/cercor/bhaa217. Cereb Cortex. 2021. PMID: 32810869 Free PMC article.
-
Threat Memory in the Sensory Cortex: Insights from Olfaction.Neuroscientist. 2024 Jun;30(3):285-293. doi: 10.1177/10738584221148994. Epub 2023 Jan 26. Neuroscientist. 2024. PMID: 36703569 Review.
-
The functional role of the medio dorsal thalamic nucleus in olfaction.Brain Res Rev. 2009 Dec 11;62(1):109-26. doi: 10.1016/j.brainresrev.2009.09.007. Epub 2009 Oct 1. Brain Res Rev. 2009. PMID: 19800366 Review.
Cited by
-
Functional Connectivity of the Chemosenses: A Review.Front Syst Neurosci. 2022 Jun 22;16:865929. doi: 10.3389/fnsys.2022.865929. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35813269 Free PMC article. Review.
-
Neurogenesis dynamics in the olfactory bulb: deciphering circuitry organization, function, and adaptive plasticity.Neural Regen Res. 2025 Jun 1;20(6):1565-1581. doi: 10.4103/NRR.NRR-D-24-00312. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934393 Free PMC article.
-
Piriform cortex is an ictogenic trigger zone in the primate brain.Epilepsia. 2025 Feb;66(2):569-582. doi: 10.1111/epi.18201. Epub 2024 Dec 5. Epilepsia. 2025. PMID: 39636294
-
Less is more: Removing a modality of an expected olfactory-visual stimulation enhances brain activation.Hum Brain Mapp. 2022 Jun 1;43(8):2567-2581. doi: 10.1002/hbm.25806. Epub 2022 Feb 10. Hum Brain Mapp. 2022. PMID: 35142405 Free PMC article.
-
Perceptual differences in olfactory fat discrimination are not detected in neural activation.Chem Senses. 2025 Jan 22;50:bjaf007. doi: 10.1093/chemse/bjaf007. Chem Senses. 2025. PMID: 39964953 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

