Adaptive responses to neurodegenerative stress in glaucoma
- PMID: 33640464
- PMCID: PMC8384979
- DOI: 10.1016/j.preteyeres.2021.100953
Adaptive responses to neurodegenerative stress in glaucoma
Abstract
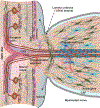
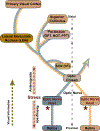
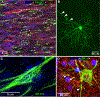
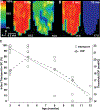
Glaucoma causes loss of vision through degeneration of the retinal ganglion cell (RGC) projection to the brain. The disease is characterized by sensitivity to intraocular pressure (IOP) conveyed at the optic nerve head, through which RGC axons pass unmyelinated to form the optic nerve. From this point, a pathogenic triumvirate comprising inflammatory, oxidative, and metabolic stress influence both proximal structures in the retina and distal structures in the optic projection. This review focuses on metabolic stress and how the optic projection may compensate through novel adaptive mechanisms to protect excitatory signaling to the brain. In the retina and proximal nerve head, the unmyelinated RGC axon segment is energy-inefficient, which leads to increased demand for adenosine-5'-triphosphate (ATP) at the risk of vulnerability to Ca2+-related metabolic and oxidative pressure. This vulnerability may underlie the bidirectional nature of progression. However, recent evidence highlights that the optic projection in glaucoma is not passive but rather demonstrates adaptive processes that may push back against neurodegeneration. In the retina, even as synaptic and dendritic pruning ensues, early progression involves enhanced excitability of RGCs. Enhancement involves depolarization of the resting membrane potential and increased response to light, independent of RGC morphological type. This response is axogenic, arising from increased levels and translocation of voltage-gated sodium channels (NaV) in the unmyelinated segment. During this same early period, large-scale networks of gap-junction coupled astrocytes redistribute metabolic resources to the optic projection stressed by elevated IOP to slow loss of axon function. This redistribution may reflect more local remodeling, as astrocyte processes respond to focal metabolic duress by boosting glycogen turnover in response to axonal activity in an effort to promote survival of the healthiest axons. Both enhanced excitability and metabolic redistribution are transient, indicating that the same adaptive mechanisms that apparently serve to slow progression ultimately may be too expensive for the system to sustain over longer periods.
Keywords: Adaptive remodeling; Astrocytes; Axon degeneration; Gap junctions; Glaucoma; Metabolic stress; Neurodegeneration; Oxidative stress.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures


References
-
- AGIS Investigators. 2000. The Advanced Glaucoma Intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol, 130:429–440. - PubMed
-
- Agostinone J, Di Polo A, 2015. Retinal ganglion cell dendrite pathology and synapse loss: Implications for glaucoma. Prog Brain Res 220, 199–216. - PubMed
-
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A, 2012. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 31, 152–181. - PubMed
-
- Ames A 3rd, 2000. CNS energy metabolism as related to function. Brain Res Brain Res Rev 34, 42–68. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

