VEGFR1 signaling in retinal angiogenesis and microinflammation
- PMID: 33640465
- PMCID: PMC8385046
- DOI: 10.1016/j.preteyeres.2021.100954
VEGFR1 signaling in retinal angiogenesis and microinflammation
Abstract
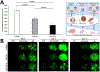
Five vascular endothelial growth factor receptor (VEGFR) ligands (VEGF-A, -B, -C, -D, and placental growth factor [PlGF]) constitute the VEGF family. VEGF-A binds VEGF receptors 1 and 2 (VEGFR1/2), whereas VEGF-B and PlGF only bind VEGFR1. Although much research has been conducted on VEGFR2 to elucidate its key role in retinal diseases, recent efforts have shown the importance and involvement of VEGFR1 and its family of ligands in angiogenesis, vascular permeability, and microinflammatory cascades within the retina. Expression of VEGFR1 depends on the microenvironment, is differentially regulated under hypoxic and inflammatory conditions, and it has been detected in retinal and choroidal endothelial cells, pericytes, retinal and choroidal mononuclear phagocytes (including microglia), Müller cells, photoreceptor cells, and the retinal pigment epithelium. Whilst the VEGF-A decoy function of VEGFR1 is well established, consequences of its direct signaling are less clear. VEGFR1 activation can affect vascular permeability and induce macrophage and microglia production of proinflammatory and proangiogenic mediators. However the ability of the VEGFR1 ligands (VEGF-A, PlGF, and VEGF-B) to compete against each other for receptor binding and to heterodimerize complicates our understanding of the relative contribution of VEGFR1 signaling alone toward the pathologic processes seen in diabetic retinopathy, retinal vascular occlusions, retinopathy of prematurity, and age-related macular degeneration. Clinically, anti-VEGF drugs have proven transformational in these pathologies and their impact on modulation of VEGFR1 signaling is still an opportunity-rich field for further research.
Keywords: Angiogenesis; Microinflammation; Placental growth factor (PlGF); Vascular endothelial growth factor receptor 1 (VEGFR1); Vascular endothelial growth factor-A (VEGF-A).
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest disclosures
Akiyoshi Uemura, Marcus Fruttiger and Patricia A. D’Amore declare no conflicts of interest. Sandro De Falco is co-founder of the startup AnBition s.r.l., Naples, Italy and co-inventor of the patents PCT/IB2018/057636, Peptides and medical uses thereof, Priority date September 11, 2019 and Italian patents n. 102018000008493 and n. 102018000008507, Peptidi ed usi medici correlati, priority date September 11, 2018. Antonia M. Joussen is a consultant for Allergan, Bayer, Novartis and Roche and has received research funding from Bayer and Novartis. Florian Sennlaub declares no conflict of interest. Lynne R. Brunck, Kristian T. Johnson, George N. Lambrou and Kay D. Rittenhouse are employees of Bayer Consumer Care AG. Thomas Langmann has participated in advisory boards from Bayer HealthCare AG.
Figures
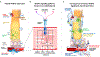



References
-
- Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK, Yeo KT, 1994. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol 118, 445–450. - PubMed
-
- Adini A, Kornaga T, Firoozbakht F, Benjamin LE, 2002. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 62, 2749–2752. - PubMed
-
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. , 1994. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med 331, 1480–1487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

