Sensitization of nerve cells to ultrasound stimulation through Piezo1-targeted microbubbles
- PMID: 33640571
- PMCID: PMC7921623
- DOI: 10.1016/j.ultsonch.2021.105494
Sensitization of nerve cells to ultrasound stimulation through Piezo1-targeted microbubbles
Abstract
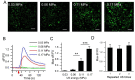
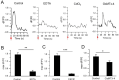
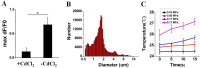
Neuromodulation by ultrasound (US) has recently drawn considerable attention due to its great advantages in noninvasiveness, high penetrability across the skull and highly focusable acoustic energy. However, the mechanisms and safety from US irradiation still remain less understood. Recently, documents revealed Piezo1, a mechanosensitive cation channel, plays key role in converting mechanical stimuli from US through its trimeric propeller-like structure. Here, we developed a Piezo1-targeted microbubble (PTMB) which can bind to the extracellular domains of Piezo1 channel. Due to the higher responsiveness of bubbles to mechanical stimuli from US, significantly lower US energy for these PTMB-binding cells may be needed to open these mechanosensitive channels. Our results showed US energy at 0.03 MPa of peak negative pressure can achieve an equivalent level of cytoplasmic Ca2+ transients which generally needs 0.17 MPa US intensity for the control cells. Cytoplasmic Ca2+ elevations were greatly reduced by chelating extracellular calcium ions or using the cationic ion channel inhibitors, confirming that US-mediated calcium influx are dependent on the Piezo1 channels. No bubble destruction and obvious temperature increase were observed during the US exposure, indicating cavitation and heating effects hardly participate in the process of Ca2+ transients. In conclusion, our study provides a novel strategy to sensitize the response of nerve cells to US stimulation, which makes it safer application for US-mediated neuromodulation in the future.
Keywords: Ca(2+) transients; Microbubbles; Neuromodulation; Piezo1; Ultrasound stimulation.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Mechanically induced integrin ligation mediates intracellular calcium signaling with single pulsating cavitation bubbles.Theranostics. 2021 Apr 7;11(12):6090-6104. doi: 10.7150/thno.56813. eCollection 2021. Theranostics. 2021. PMID: 33897901 Free PMC article.
-
Activation of Piezo1 mechanosensitive ion channel in HEK293T cells by 30 MHz vertically deployed surface acoustic waves.Biochem Biophys Res Commun. 2019 Oct 20;518(3):541-547. doi: 10.1016/j.bbrc.2019.08.078. Epub 2019 Aug 23. Biochem Biophys Res Commun. 2019. PMID: 31451220 Free PMC article.
-
The mechanosensitive ion channel Piezo1 contributes to ultrasound neuromodulation.Proc Natl Acad Sci U S A. 2023 May 2;120(18):e2300291120. doi: 10.1073/pnas.2300291120. Epub 2023 Apr 25. Proc Natl Acad Sci U S A. 2023. PMID: 37098060 Free PMC article.
-
Immunoregulatory Role of the Mechanosensitive Ion Channel Piezo1 in Inflammation and Cancer.Molecules. 2022 Dec 26;28(1):213. doi: 10.3390/molecules28010213. Molecules. 2022. PMID: 36615408 Free PMC article. Review.
-
Mechanosensing by Piezo1 and its implications for physiology and various pathologies.Biol Rev Camb Philos Soc. 2022 Apr;97(2):604-614. doi: 10.1111/brv.12814. Epub 2021 Nov 15. Biol Rev Camb Philos Soc. 2022. PMID: 34781417 Review.
Cited by
-
Nanoparticles carrying paclitaxel and anti-miR-221 for breast cancer therapy triggered by ultrasound.Cell Death Discov. 2023 Aug 15;9(1):298. doi: 10.1038/s41420-023-01594-9. Cell Death Discov. 2023. PMID: 37582832 Free PMC article.
-
Genetics-Based Targeting Strategies for Precise Neuromodulation.Adv Sci (Weinh). 2025 Jul;12(28):e13817. doi: 10.1002/advs.202413817. Epub 2025 May 19. Adv Sci (Weinh). 2025. PMID: 40387259 Free PMC article. Review.
-
Force From Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels.Front Cell Dev Biol. 2022 May 2;10:886048. doi: 10.3389/fcell.2022.886048. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35586339 Free PMC article. Review.
-
The Role of the Piezo1 Mechanosensitive Channel in Heart Failure.Curr Issues Mol Biol. 2023 Jul 13;45(7):5830-5848. doi: 10.3390/cimb45070369. Curr Issues Mol Biol. 2023. PMID: 37504285 Free PMC article. Review.
-
Transcranial focused ultrasound precise neuromodulation: a review of focal size regulation, treatment efficiency and mechanisms.Front Neurosci. 2024 Sep 5;18:1463038. doi: 10.3389/fnins.2024.1463038. eCollection 2024. Front Neurosci. 2024. PMID: 39301015 Free PMC article. Review.
References
-
- Demene C., Baranger J., Bernal M., Delanoe C., Auvin S., Biran V., Alison M., Mairesse J., Harribaud E., Pernot M., Tanter M., Baud O. Sci. Transl. Med. 2017;9:eaah6756. - PubMed
-
- Alamri A., Ughratdar I., Samuel M., Ashkan K. Br. J. Neurosurg. 2015;29:319–328. - PubMed
-
- van de Ruit M., Perenboom M.J., Grey M.J. Brain. Stimul. 2015;8:231–239. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

