Female rats display greater nicotine withdrawal-induced cellular activation of a central portion of the interpeduncular nucleus versus males: A study of Fos immunoreactivity within provisionally assigned interpeduncular subnuclei
- PMID: 33640680
- PMCID: PMC8043600
- DOI: 10.1016/j.drugalcdep.2021.108640
Female rats display greater nicotine withdrawal-induced cellular activation of a central portion of the interpeduncular nucleus versus males: A study of Fos immunoreactivity within provisionally assigned interpeduncular subnuclei
Abstract
Background: The interpeduncular nucleus (>1840) (IPN) has been shown to modulate the behavioral effects of nicotine withdrawal in male rodents. To date, the contribution of this brain structure to sex differences in withdrawal is largely unexplored.
Methods: This study compared neuronal activation, as reported by observable Fos expression in the IPN of nicotine-dependent female and male rats experiencing withdrawal. We provisionally localized the Fos-expressing cells to certain IPN subnuclei within Swanson's standardized brain atlas (2018). Adult female and male rats were prepared with a pump that delivered nicotine (3.2 mg/kg/day; base) continuously. Controls received a sham surgery. Fourteen days later, the rats received administration of saline or the nicotinic receptor antagonist, mecamylamine (3.0 mg/kg; salt), and physical signs and anxiety-like behavior were assessed. The rats were then euthanized and brain sections containing the IPN were processed for Fos immunofluorescence to infer the possible IPN subnuclei displaying differential activation between sexes.
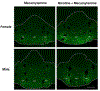
Results: Both female and male rats displayed withdrawal-induced Fos expression within the IPN. Compared to males, female rats displayed greater numbers of withdrawal-induced Fos-positive cells within a circumscribed portion of the IPN that may fall within the cytoarchitectural boundaries of the central subnucleus (>1840) (IPNc). The withdrawal-induced activation of the IPN was correlated with negative affective states in females, but not males.
Conclusion: These data suggest that a centrally located group of IPN cells, presumably situated partly or completely within the IPNc, play a role in modulating sex differences in negative affective states produced by withdrawal.
Keywords: Dependence; Immunofluorescence; Sex differences; Standardized brain atlas; c-Fos.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
No conflict declared.
Figures

Similar articles
-
Sex differences in cholinergic systems in the interpeduncular nucleus following nicotine exposure and withdrawal.Neuropharmacology. 2019 Nov 1;158:107714. doi: 10.1016/j.neuropharm.2019.107714. Epub 2019 Jul 17. Neuropharmacology. 2019. PMID: 31325431 Free PMC article.
-
Dynamic activity of interpeduncular nucleus GABAergic neurons controls expression of nicotine withdrawal in male mice.Neuropsychopharmacology. 2022 Feb;47(3):641-651. doi: 10.1038/s41386-021-01107-1. Epub 2021 Jul 29. Neuropsychopharmacology. 2022. PMID: 34326477 Free PMC article.
-
Amino acid systems in the interpeduncular nucleus are altered in a sex-dependent manner during nicotine withdrawal.J Neurosci Res. 2022 Aug;100(8):1573-1584. doi: 10.1002/jnr.24826. Epub 2021 Mar 22. J Neurosci Res. 2022. PMID: 33751631 Free PMC article.
-
The habenulo-interpeduncular pathway in nicotine aversion and withdrawal.Neuropharmacology. 2015 Sep;96(Pt B):213-22. doi: 10.1016/j.neuropharm.2014.11.019. Epub 2014 Dec 2. Neuropharmacology. 2015. PMID: 25476971 Free PMC article. Review.
-
The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation.J Neurochem. 2017 Aug;142 Suppl 2(Suppl 2):130-143. doi: 10.1111/jnc.14008. J Neurochem. 2017. PMID: 28791703 Free PMC article. Review.
Cited by
-
A neuronal coping mechanism linking stress-induced anxiety to motivation for reward.Sci Adv. 2023 Dec 8;9(49):eadh9620. doi: 10.1126/sciadv.adh9620. Epub 2023 Dec 6. Sci Adv. 2023. PMID: 38055830 Free PMC article.
-
Nicotine Withdrawal Drives Aversive Behaviors by Recruiting Inhibitory Interpeduncular Nucleus Inputs to the Laterodorsal Tegmentum in Mice.J Neurosci. 2025 Aug 6;45(32):e2405242025. doi: 10.1523/JNEUROSCI.2405-24.2025. J Neurosci. 2025. PMID: 40659528 Free PMC article.
-
Effects of E-Cigs on Physiological Pathways and Proposed Therapeutic Intervention with Bixin.Biomedicines. 2024 Nov 27;12(12):2705. doi: 10.3390/biomedicines12122705. Biomedicines. 2024. PMID: 39767612 Free PMC article. Review.
-
Brain Magnetic Resonance Imaging Features of Nicotine-Dependent Individuals and Its Correlation with Polymorphisms of Dopamine D Receptor Gene.Contrast Media Mol Imaging. 2022 Aug 24;2022:2296776. doi: 10.1155/2022/2296776. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 36082055 Free PMC article.
-
The influence of ovarian hormones on the putative mechanisms that promote female nicotine use.Curr Opin Neurobiol. 2024 Oct;88:102900. doi: 10.1016/j.conb.2024.102900. Epub 2024 Aug 16. Curr Opin Neurobiol. 2024. PMID: 39153250 Free PMC article. Review.
References
-
- Baer K.E. von, 1837. Über Entwickelungsgeschichte der Thiere. Beobachtung und Reflexion, Bornträger (Koch, Königsberg). Available online at the Biodiversity Heritage Library: 10.5962/bhl.title.6303 - DOI
-
- Cajal SR, 1895. Apuntes para el estudio del bulbo raquídeo, cerebelo y origen de los nervios encefálicos. Anal Soc Esp Hist Nat 24, 5–253. Available online at the Biodiversity Heritage Library: https://www.biodiversitylibrary.org/page/16176071. Last accessed on 21 Aug 2020.
-
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F, 1987. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neurosci. 21 (1), 253–270. 10.1016/0306-4522(87)90337-x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

