Maf1 suppression of ATF5-dependent mitochondrial unfolded protein response contributes to rapamycin-induced radio-sensitivity in lung cancer cell line A549
- PMID: 33640883
- PMCID: PMC7993702
- DOI: 10.18632/aging.202584
Maf1 suppression of ATF5-dependent mitochondrial unfolded protein response contributes to rapamycin-induced radio-sensitivity in lung cancer cell line A549
Abstract
mTOR is well known to promote tumor growth but its roles in enhancing chemotherapy and radiotherapy have not been well studied. mTOR inhibition by rapamycin can sensitize cancer cells to radiotherapy. Here we show that Maf1 is required for rapamycin to increase radio-sensitivity in A549 lung cancer cells. In response to ionizing radiation (IR), Maf1 is inhibited by Akt-dependent re-phosphorylation, which activates mitochondrial unfolded protein response (UPRmt) through ATF5. Rapamycin suppresses IR-induced Maf1 re-phosphorylation and UPRmt activation in A549 cells, resulting in increased sensitivity to IR-mediated cytotoxicity. Consistently, Maf1 knockdown activates ATF5-transcription of mtHSP70 and HSP60, enhances mitochondrial membrane potential, reduces intracellular ROS levels and dampens rapamycin's effect on increasing IR-mediated cytotoxicity. In addition, Maf1 overexpression suppresses ethidium bromide-induced UPRmt and enhances IR-mediated cytotoxicity. Supporting our cell-based studies, elevated expression of UPRmt makers (mtHSP70 and HSP60) are associated with poor prognosis in patients with lung adenocarcinoma (LAUD). Together, our study reveals a novel role of Maf1-UPRmt axis in mediating rapamycin's enhancing effect on IR sensitivity in A549 lung cancer cells.
Keywords: Maf1; mTOR; mitochondrial unfolded protein response; non-small cell lung cancer cell; radio-resistance.
Conflict of interest statement
Figures

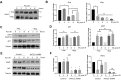
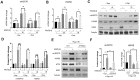
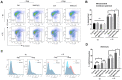

References
-
- Liang SQ, Bührer ED, Berezowska S, Marti TM, Xu D, Froment L, Yang H, Hall SR, Vassella E, Yang Z, Kocher GJ, Amrein MA, Riether C, et al. mTOR mediates a mechanism of resistance to chemotherapy and defines a rational combination strategy to treat KRAS-mutant lung cancer. Oncogene. 2019; 38:622–36. 10.1038/s41388-018-0479-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

