Docking domain-mediated subunit interactions in natural product megasynth(et)ases
- PMID: 33640957
- PMCID: PMC9113145
- DOI: 10.1093/jimb/kuab018
Docking domain-mediated subunit interactions in natural product megasynth(et)ases
Abstract
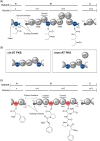
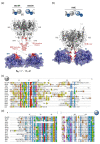
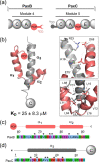
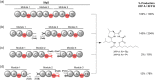
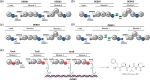
Polyketide synthase (PKS) and non-ribosomal peptide synthetase (NRPS) multienzymes produce numerous high value metabolites. The protein subunits which constitute these megasynth(et)ases must undergo ordered self-assembly to ensure correct organisation of catalytic domains for the biosynthesis of a given natural product. Short amino acid regions at the N- and C-termini of each subunit, termed docking domains (DDs), often occur in complementary pairs, which interact to facilitate substrate transfer and maintain pathway fidelity. This review details all structurally characterised examples of NRPS and PKS DDs to date and summarises efforts to utilise DDs for the engineering of biosynthetic pathways.
Keywords: Biosynthesis; Non-ribosomal peptide synthetase; Polyketide synthase.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Figures





References
-
- Aparicio J. F., Molnár I., Schwecke T., König A., Haydock S. F., Ee Khaw L., Staunton J., Leadlay P. F. (1996). Organization of the biosynthetic gene cluster for rapamycin in Streptomyces hygroscopicus: Analysis of the enzymatic domains in the modular polyketide synthase. Gene, 169(1), 9–16. https://doi.org/10.1016/0378-1119(95)00800-4. - PubMed
-
- Bretschneider T., Heim J. B., Heine D., Winkler R., Busch B., Kusebauch B., Stehle T., Zocher G., Hertweck C. (2013). Vinylogous chain branching catalysed by a dedicated polyketide synthase module. Nature, 502(7469), 124–128. https://doi.org/10.1038/nature12588. - PubMed
-
- Broadhurst R. W., Nietlispach D., Wheatcroft M. P., Leadlay P. F., Weissman K. J. (2003). The structure of docking domains in modular polyketide synthases. Chemistry & Biology, 10(8), 723–731. https://doi.org/10.1016/s1074-5521(03)00156-x. - PubMed
-
- Buchholz T. J., Geders T. W., Bartley F. E., Reynolds K. A., Smith J. L., Sherman D. H. (2009). Structural basis for binding specificity between subclasses of modular polyketide synthase docking domains. ACS Chemical Biology, 4(1), 41–52. https://doi.org/10.1021/cb8002607. - PMC - PubMed
-
- Cai X., Zhao L., Bode H. B. (2019). Reprogramming promiscuous nonribosomal peptide synthetases for production of specific peptides. Organic Letters, 21(7), 2116–2120. https://doi.org/10.1021/acs.orglett.9b00395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

