Studies of transmembrane peptides by pulse dipolar spectroscopy with semi-rigid TOPP spin labels
- PMID: 33640998
- PMCID: PMC8071797
- DOI: 10.1007/s00249-021-01508-6
Studies of transmembrane peptides by pulse dipolar spectroscopy with semi-rigid TOPP spin labels
Abstract
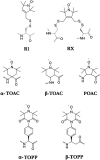
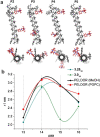
Electron paramagnetic resonance (EPR)-based pulsed dipolar spectroscopy measures the dipolar interaction between paramagnetic centers that are separated by distances in the range of about 1.5-10 nm. Its application to transmembrane (TM) peptides in combination with modern spin labelling techniques provides a valuable tool to study peptide-to-lipid interactions at a molecular level, which permits access to key parameters characterizing the structural adaptation of model peptides incorporated in natural membranes. In this mini-review, we summarize our approach for distance and orientation measurements in lipid environment using novel semi-rigid TOPP [4-(3,3,5,5-tetramethyl-2,6-dioxo-4-oxylpiperazin-1-yl)-L-phenylglycine] labels specifically designed for incorporation in TM peptides. TOPP labels can report single peak distance distributions with sub-angstrom resolution, thus offering new capabilities for a variety of TM peptide investigations, such as monitoring of various helix conformations or measuring of tilt angles in membranes.
Keywords: DEER; Dipolar spectroscopy; PDS; PELDOR; Pulsed ESR; SDSL; Spin label; Transmembrane peptide; α-TOPP; β-TOPP; β-peptide.
Figures






Similar articles
-
Pulse EPR Measurements of Intramolecular Distances in a TOPP-Labeled Transmembrane Peptide in Lipids.Biophys J. 2016 Dec 6;111(11):2345-2348. doi: 10.1016/j.bpj.2016.10.022. Epub 2016 Nov 9. Biophys J. 2016. PMID: 27836102 Free PMC article.
-
Semi-Rigid Nitroxide Spin Label for Long-Range EPR Distance Measurements of Lipid Bilayer Embedded β-Peptides.Chemistry. 2019 Feb 11;25(9):2203-2207. doi: 10.1002/chem.201805880. Epub 2019 Jan 16. Chemistry. 2019. PMID: 30500095
-
PELDOR Measurements on Nitroxide-Labeled Oligonucleotides.Methods Mol Biol. 2022;2439:241-274. doi: 10.1007/978-1-0716-2047-2_16. Methods Mol Biol. 2022. PMID: 35226326
-
Orthogonal spin labeling and pulsed dipolar spectroscopy for protein studies.Methods Enzymol. 2022;666:79-119. doi: 10.1016/bs.mie.2022.02.004. Epub 2022 Mar 17. Methods Enzymol. 2022. PMID: 35465930 Review.
-
Pulsed electron-electron double resonance: beyond nanometre distance measurements on biomacromolecules.Biochem J. 2011 Mar 15;434(3):353-63. doi: 10.1042/BJ20101871. Biochem J. 2011. PMID: 21348855 Review.
Cited by
-
Special issue: Multicomponent lipid membranes-how molecular organisation leads to function.Eur Biophys J. 2021 Mar;50(2):107-108. doi: 10.1007/s00249-021-01535-3. Eur Biophys J. 2021. PMID: 33860333 Free PMC article. No abstract available.
-
Structure and dynamics determine G protein coupling specificity at a class A GPCR.Sci Adv. 2025 Mar 21;11(12):eadq3971. doi: 10.1126/sciadv.adq3971. Epub 2025 Mar 19. Sci Adv. 2025. PMID: 40106559 Free PMC article.
-
Expanding the Diversity of Nitroxide-Based Paramagnetic Probes Conjugated to Non-Canonical Amino Acids for Sdsl-Epr Applications.Chembiochem. 2025 Apr 1;26(7):e202500064. doi: 10.1002/cbic.202500064. Epub 2025 Mar 12. Chembiochem. 2025. PMID: 40011209 Free PMC article.
-
A novel approach to modeling side chain ensembles of the bifunctional spin label RX.bioRxiv [Preprint]. 2023 May 24:2023.05.24.542139. doi: 10.1101/2023.05.24.542139. bioRxiv. 2023. PMID: 37292623 Free PMC article. Preprint.
References
-
- Appella DH, Christianson LA, Karle IL, Powell DR, Gellman SH. β-Peptide foldamers: robust helix formation in a new family of β-amino acid oligomers. J Am Chem Soc. 1996;118:13071–13072. doi: 10.1021/ja963290l. - DOI
-
- Arvidsson PI, Rueping M, Seebach D (2001) Design, machine synthesis, and NMR-solution structure of a β-heptapeptide forming a salt-bridge stabilised 3-helix in methanol and in water. Chem Commun 649–650. 10.1039/B101085I
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

