Heterogeneity in α-synuclein fibril activity correlates to disease phenotypes in Lewy body dementia
- PMID: 33641009
- PMCID: PMC8055043
- DOI: 10.1007/s00401-021-02288-1
Heterogeneity in α-synuclein fibril activity correlates to disease phenotypes in Lewy body dementia
Abstract
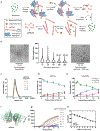
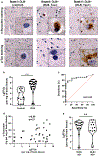
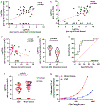
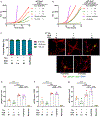
α-Synuclein aggregation underlies pathological changes in Lewy body dementia. Recent studies highlight structural variabilities associated with α-synuclein aggregates in patient populations. Here, we develop a quantitative real-time quaking-induced conversion (qRT-QuIC) assay to measure permissive α-synuclein fibril-templating activity in tissues and cerebrospinal fluid (CSF). The assay is anchored through reference panels of stabilized ultra-short fibril particles. In humanized α-synuclein transgenic mice, qRT-QuIC identifies differential levels of fibril activity across the brain months before the deposition of phosphorylated α-synuclein in susceptible neurons. α-Synuclein fibril activity in cortical brain extracts from dementia with Lewy bodies (DLB) correlates with activity in matched ventricular CSF. Elevated α-synuclein fibril activity in CSF corresponds to reduced survival in DLB. α-Synuclein fibril particles amplified from cases with high fibril activity show superior templating in the formation of new inclusions in neurons relative to the same number of fibril particles amplified from DLB cases with low fibril activity. Our results highlight a previously unknown broad heterogeneity of fibril-templating activities in DLB that may contribute to disease phenotypes. We predict that quantitative assessments of fibril activities in CSF that correlate to fibril activities in brain tissue will help stratify patient populations as well as measure therapeutic responses to facilitate the development of α-synuclein-targeted therapeutics.
Keywords: Aggregation; Biomarker; Neurodegeneration; Parkinson’s disease; Prion; SNCA.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

