Proximal Tubule-Specific Deletion of Angiotensin II Type 1a Receptors in the Kidney Attenuates Circulating and Intratubular Angiotensin II-Induced Hypertension in PT- Agtr1a-/- Mice
- PMID: 33641366
- PMCID: PMC7946728
- DOI: 10.1161/HYPERTENSIONAHA.120.16336
Proximal Tubule-Specific Deletion of Angiotensin II Type 1a Receptors in the Kidney Attenuates Circulating and Intratubular Angiotensin II-Induced Hypertension in PT- Agtr1a-/- Mice
Abstract
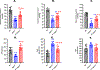
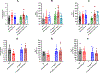
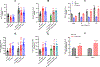
[Figure: see text].
Keywords: angiotensin II; electrolytes; homeostasis; hypertension; natriuresis.
Conflict of interest statement
Disclosures of Conflict of Interest
None.
Figures

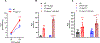

Similar articles
-
Angiotensin II and AT1a Receptors in the Proximal Tubules of the Kidney: New Roles in Blood Pressure Control and Hypertension.Int J Mol Sci. 2022 Feb 22;23(5):2402. doi: 10.3390/ijms23052402. Int J Mol Sci. 2022. PMID: 35269547 Free PMC article. Review.
-
Genetic Deletion of AT1a Receptor or Na+/H+ Exchanger 3 Selectively in the Proximal Tubules of the Kidney Attenuates Two-Kidney, One-Clip Goldblatt Hypertension in Mice.Int J Mol Sci. 2022 Dec 13;23(24):15798. doi: 10.3390/ijms232415798. Int J Mol Sci. 2022. PMID: 36555438 Free PMC article.
-
Sex differences in angiotensin II-induced hypertension and kidney injury: role of AT1a receptors in the proximal tubule of the kidney.Clin Sci (Lond). 2021 Aug 13;135(15):1825-1843. doi: 10.1042/CS20201574. Clin Sci (Lond). 2021. PMID: 34282828 Free PMC article.
-
Deletion of AT1a receptors selectively in the proximal tubules of the kidney alters the hypotensive and natriuretic response to atrial natriuretic peptide via NPRA/cGMP/NO signaling.Am J Physiol Renal Physiol. 2024 Dec 1;327(6):F946-F956. doi: 10.1152/ajprenal.00160.2024. Epub 2024 Oct 3. Am J Physiol Renal Physiol. 2024. PMID: 39361722
-
AT2 Receptors: Potential Therapeutic Targets for Hypertension.Am J Hypertens. 2017 Apr 1;30(4):339-347. doi: 10.1093/ajh/hpw121. Am J Hypertens. 2017. PMID: 27664954 Review.
Cited by
-
Intracellular Angiotensin II Stimulation of Sodium Transporter Expression in Proximal Tubule Cells via AT1 (AT1a) Receptor-Mediated, MAP Kinases ERK1/2- and NF-кB-Dependent Signaling Pathways.Cells. 2023 May 28;12(11):1492. doi: 10.3390/cells12111492. Cells. 2023. PMID: 37296613 Free PMC article.
-
Role of Angiotensin II Type 1a Receptor (AT1aR) of Renal Tubules in Regulating Inwardly Rectifying Potassium Channels 4.2 (Kir4.2), Kir4.1, and Epithelial Na+ Channel (ENaC).Hypertension. 2024 Jan;81(1):126-137. doi: 10.1161/HYPERTENSIONAHA.123.21389. Epub 2023 Nov 1. Hypertension. 2024. PMID: 37909221 Free PMC article.
-
Kidney and blood pressure regulation-latest evidence for molecular mechanisms.Clin Kidney J. 2023 Jan 24;16(6):952-964. doi: 10.1093/ckj/sfad015. eCollection 2023 Jun. Clin Kidney J. 2023. PMID: 37261007 Free PMC article. Review.
-
Angiotensin II and AT1a Receptors in the Proximal Tubules of the Kidney: New Roles in Blood Pressure Control and Hypertension.Int J Mol Sci. 2022 Feb 22;23(5):2402. doi: 10.3390/ijms23052402. Int J Mol Sci. 2022. PMID: 35269547 Free PMC article. Review.
-
Long-Term Sodium Deficiency Reduces Sodium Excretion but Impairs Renal Function and Increases Stone Formation in Hyperoxaluric Calcium Oxalate Rats.Int J Mol Sci. 2024 Apr 1;25(7):3942. doi: 10.3390/ijms25073942. Int J Mol Sci. 2024. PMID: 38612752 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

