Validation of Quality Control Parameters of Cassette-Based Gallium-68-DOTA-Tyr3-Octreotate Synthesis
- PMID: 33642752
- PMCID: PMC7905293
- DOI: 10.4103/ijnm.IJNM_66_20
Validation of Quality Control Parameters of Cassette-Based Gallium-68-DOTA-Tyr3-Octreotate Synthesis
Abstract
Purpose of the study: Gallium (Ga)-68-DOTA peptides targeting somatostatin receptors have been assessed as a valuable tool in neuroendocrine tumor imaging using positron emission tomography. However, at the moment, a specific monograph in the European Pharmacopoeia (Ph. Eur.) does exist only for Ga-68-edotreotide (DOTATOC) injection. Here, we report on the validation process of Ga-68-DOTA-Tyr3-octreotate (DOTATATE) cassette-based production and quality control (QC).
Materials and methods: Preparation of Ga-68-DOTATATE was performed according to the current European Union-good manufacturing practices, the current good radiopharmacy practice, the Ph. Eur., and the guidelines on validation of analytical methods for radiopharmaceuticals. Process was validated via three consecutive production runs to ensure that the methods are reproducible and reliable in routine use. The QC tests for Ga-68-DOTATATE were radiochemical purity (RCP - high-pressure liquid chromatography [HPLC]), radiochemical impurities 68Ga3+ (HPLC and instant thin layer chromatography [ITLC]), chemical purity (HPLC and gas chromatography [GC]), pH (pH-strips), radionuclidic purity (principal γ-photon), germanium-breakthrough (68Ge-content), Ga-68 half-life (γ-ray spectrometry), and sterility/endotoxin assay.
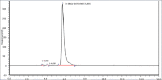
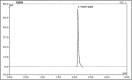
Results: Radiolabeling procedure of Ga-68-DOTATATE fits all the applicable Ph. Eur. specifications. RCP measured via ITLC was >99% in the three validation batches. HPLC-measured RCP resulted 99.45%, 99.78%, and 99.75%. Germanium-breakthrough was far below the recommended level established in the Ph. Eur. Ga-68-DOTATOC injection (#2482). Residual ethanol tested with GC was less than 10%. All the batches were tested for endotoxin content, which always resulted lower than 17.5 EU/ml. All preparations passed the sterility tests. pH of the final product was 7 in all samples.
Conclusion: Ga-68-DOTATATE fulfilled all the pre-set QCs and release criteria in the batches considered for this validation study. The results demonstrated a batch-to-batch reproducibility, ensuring that synthesis process leads to the expected final product in terms of yield, quality, reliability, safety, and efficacy.
Keywords: Gallium-68; positron emission tomography; radiopharmaceutical validation; somatostatin receptors.
Copyright: © 2020 Indian Journal of Nuclear Medicine.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, et al. Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer. 2008;113:2655–64. - PubMed
-
- Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, et al. Internalization of SST2, SST3, and SST5 receptors: Effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47:502–11. - PubMed
-
- Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor SST1-SST5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28:836–46. - PubMed
-
- Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. - PubMed
-
- Gallium (68 Ga) chloride solution for radiolabelling. European Directorate for the quality of medicines. Eur Pharmacopeia. 2013;2464:1060–1.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
