Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer
- PMID: 33642753
- PMCID: PMC7905268
- DOI: 10.4103/ijnm.IJNM_130_20
Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer
Abstract
Aims: Actinium-225 (225Ac) labeled prostate-specific membrane antigen (PSMA)-617 is a novel treatment modality in the management of metastatic castration-resistant prostate cancer (mCRPC). The present study was conducted to assess the impact of 225Ac-PSMA-617 therapy on the quality-of-life of patients with heavily pretreated mCRPC using the National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy-Prostate Symptom Index-17 (NCCN-FACT-FPSI-17) questionnaire.
Materials and methods: This was a retrospective single-center study where data of consecutive heavily pretreated mCRPC patients treated with 225Ac-PSMA-617 from January 2019 to February 2020, was collected and analyzed for the biochemical response, quality-of-life outcomes and treatment-related toxicity.
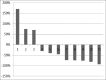
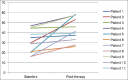
Results: Eleven heavily pretreated mCRPC patients received a median cumulative dose of 8.3 MBq (interquartile range [IQR] 5.6-20.4 MBq) 225Ac-PSMA-617 over 1-4 cycles. 5/11 patients (46%) showed a ≥50% decline in Prostate Specific Antigen (PSA), while stable values and PSA progression were observed in 3/11 (27%) patients each. Pre- and post-therapy NCCN-FACT-FPSI-17 questionnaires revealed statistically significant improvement in the total FPSI score (P = 0.003) as well as the disease-related symptoms-physical (P = 0.004) and disease-related symptoms-emotional (P = 0.046) subscores. Among the physical symptoms, significant improvement was noted with respect to pain, difficulty in urination, bone pain, fatigue, and restriction in physical activity. No significant change was noted in the treatment side-effects subscore. Of the treatment-related adverse effects, Grade 3 dryness of the mouth, anemia, and nephrotoxicity was observed in 1/11 patients (9%) each and Grade 3 thrombocytopenia in 2/11 patients (18%).
Conclusion: Health-related quality-of-life of the mCRPC patients improved significantly with 225Ac-PSMA-617 despite extensive pretreatment and advanced nature of the disease.
Keywords: Actinium-225-prostate-specific membrane antigen-617; castration-resistant prostate cancer; prostate-specific membrane antigen; quality-of-life.
Copyright: © 2020 Indian Journal of Nuclear Medicine.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA.Eur Urol. 2021 Mar;79(3):343-350. doi: 10.1016/j.eururo.2020.11.013. Epub 2020 Dec 5. Eur Urol. 2021. PMID: 33293081
-
225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study.Eur J Nucl Med Mol Imaging. 2019 Jan;46(1):129-138. doi: 10.1007/s00259-018-4167-0. Epub 2018 Sep 19. Eur J Nucl Med Mol Imaging. 2019. PMID: 30232539 Free PMC article.
-
Clinical outcomes and molecular profiling of advanced metastatic castration-resistant prostate cancer patients treated with 225Ac-PSMA-617 targeted alpha-radiation therapy.Urol Oncol. 2021 Oct;39(10):729.e7-729.e16. doi: 10.1016/j.urolonc.2020.12.002. Epub 2021 Jan 11. Urol Oncol. 2021. PMID: 33353867
-
225 Ac-PSMA-617-targeted alpha therapy for the treatment of metastatic castration-resistant prostate cancer: A systematic review and meta-analysis.Prostate. 2021 Jun;81(9):580-591. doi: 10.1002/pros.24137. Epub 2021 Apr 27. Prostate. 2021. PMID: 33905559
-
Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer-a systematic review and meta-analysis.Prostate Cancer Prostatic Dis. 2021 Sep;24(3):880-890. doi: 10.1038/s41391-021-00349-w. Epub 2021 Mar 21. Prostate Cancer Prostatic Dis. 2021. PMID: 33746213
Cited by
-
Efficacy and Safety of [225Ac]Ac-PSMA-617 Augmented [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis.Pharmaceutics. 2021 May 14;13(5):722. doi: 10.3390/pharmaceutics13050722. Pharmaceutics. 2021. PMID: 34069003 Free PMC article.
-
Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis.Med Princ Pract. 2023;32(3):178-191. doi: 10.1159/000531246. Epub 2023 May 29. Med Princ Pract. 2023. PMID: 37247612 Free PMC article.
-
Recent Innovations and Nano-Delivery of Actinium-225: A Narrative Review.Pharmaceutics. 2023 Jun 13;15(6):1719. doi: 10.3390/pharmaceutics15061719. Pharmaceutics. 2023. PMID: 37376167 Free PMC article. Review.
-
Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis.Front Oncol. 2022 Feb 3;12:796657. doi: 10.3389/fonc.2022.796657. eCollection 2022. Front Oncol. 2022. PMID: 35186737 Free PMC article.
-
The current status of prostate cancer treatment and PSMA theranostics.Ther Adv Med Oncol. 2023 Jul 3;15:17588359231182293. doi: 10.1177/17588359231182293. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37424944 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Klaff R, Rosell J, Varenhorst E, Sandblom G. The long-term disease-specific mortality of low-risk localized prostate cancer: A prospective population-based register study over two decades. Urology. 2016;91:77–82. - PubMed
-
- Helgstrand JT, Røder MA, Klemann N, Toft BG, Lichtensztajn DY, Brooks JD, et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer – A population-based analysis of 2 national cohorts. Cancer. 2018;124:2931–8. - PubMed
-
- Mottet N, Cornford P, van den Bergh RC, Briers E, De Santis M, Fanti S, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer EAU Guidelines Office. 2019
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous