Prevalence and phenotypic characterization of Salmonella enterica isolates from three species of wild marine turtles in Grenada, West Indies
- PMID: 33642807
- PMCID: PMC7896897
- DOI: 10.14202/vetworld.2021.222-229
Prevalence and phenotypic characterization of Salmonella enterica isolates from three species of wild marine turtles in Grenada, West Indies
Abstract
Background and aim: Salmonella enterica causes enteric disease in mammals and may potentially be transmitted from marine turtles that shed the pathogen in the environment. Marine turtle-associated human salmonellosis is a potential public health concern in Grenada, as the island supports populations of leatherback turtles (Dermochelys coriacea), hawksbill turtles (Eretmochelys imbricata), and green turtles (Chelonia mydas) that interface with veterinarians and conservation workers, the local population, and the thousands of visitors that frequent the island yearly. To date, the prevalence of S. enterica has only been examined in a small subset of marine turtles in the Caribbean and no studies have been conducted in Grenada. The aim of this study was to quantify the prevalence of S. enterica in leatherback, hawksbill and green turtles in Grenada, characterize phenotypes and DNA profiles, and explore the potential risk to human health in the region.
Materials and methods: A total of 102 cloacal swabs were obtained from nesting leatherback turtles and foraging hawksbill and green turtles. Samples were cultured on enrichment and selective media and isolates were phenotypically characterized using serotyping, pulsed-phase gel electrophoresis, and antibiotic susceptibility. Enrichment broths were additionally screened by polymerase chain reaction (PCR) using S. enterica-specific primers.
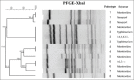
Results: S. enterica was cultured from 15/57 (26.3%) leatherback turtles, 0/28 hawksbill, and 0/17 green turtles. This included S. enterica serovars Montevideo, S. I:4,5,12:i:-, Salmonella Typhimurium, Salmonella Newport, S. I:6,7:-:-, and S. I:4,5,12:-:-. Five/15 leatherback turtles carried multiple serovars. Eight pulsotype groups were identified with multiple clustering; however, there was no clear association between pulsotype group and serotype profile. Five/71 isolates showed resistance to streptomycin or ampicillin. Twenty-one/57 leatherback turtles, 14/28 hawksbill turtles, and 8/17 green turtles tested positive for S. enterica by quantitative PCR.
Conclusion: Nesting leatherback turtles actively shed S. enterica and poses a risk for zoonosis; however, the presence of viable pathogen in green and hawksbill species is unclear. These findings help elucidate the role of marine turtles as potential sources of zoonotic S. enterica and provide baseline data for one health research in Grenada and the wider Caribbean region.
Keywords: Salmonella enterica; antimicrobials; marine turtles; pulsotypes; serotypes; zoonosis.
Copyright: © Edwards, et al.
Figures
References
-
- Majowicz S.E, Musto J, Scallan E, Angulo F.J, Kirk M, O'Brien S.J, Jones T.F, Fazil A, Hoekstra R.M. The global burden of non-typhoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50(6):882–889. - PubMed
-
- Eng S, Pusparajah P, Mutalib N.A, Ser H, Chan K, Lee L. Salmonella:A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8(3):284–293.
-
- Demirbilek S.K. Salmonella:A re-emerging pathogen. In: Mascellino MT, editor. Salmonellosis in Animals. Vol. 2. London: IntechOpen; 2017. pp. 19–31.
-
- Schoeni J.L, Glass K, McDermott J.L, Wong C.L. Growth and penetration of Salmonella Enteritidis, Salmonella Heidelberg and Salmonella Typhimurium in eggs. Food Microbiol. 1995;24(3):385–396. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources