Tau Pathology and Adult Hippocampal Neurogenesis: What Tau Mouse Models Tell us?
- PMID: 33643196
- PMCID: PMC7902892
- DOI: 10.3389/fneur.2021.610330
Tau Pathology and Adult Hippocampal Neurogenesis: What Tau Mouse Models Tell us?
Abstract
Adult hippocampal neurogenesis (AHN) has been widely confirmed in mammalian brains. A growing body of evidence points to the fact that AHN sustains hippocampal-dependent functions such as learning and memory. Impaired AHN has been reported in post-mortem human brain hippocampus of Alzheimer's disease (AD) and is considered to contribute to defects in learning and memory. Neurofibrillary tangles (NFTs) and amyloid plaques are the two key neuropathological hallmarks of AD. NFTs are composed of abnormal tau proteins accumulating in many brain areas during the progression of the disease, including in the hippocampus. The physiological role of tau and impact of tau pathology on AHN is still poorly understood. Modifications in AHN have also been reported in some tau transgenic and tau-deleted mouse models. We present here a brief review of advances in the relationship between development of tau pathology and AHN in AD and what insights have been gained from studies in tau mouse models.
Keywords: Alzheimer's disease; dentate gyrus; neurogenesis; tau; tauopathy.
Copyright © 2021 Houben, Homa, Yilmaz, Leroy, Brion and Ando.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
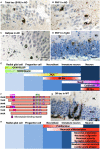
Figures
References
-
- Brion JP, Passareiro H, Nunez J, Flament-Durand J. Mise en évidence immunologique de la protéine tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d'Alzheimer. Arch Biol. (1985) 95:229–35.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

