Reciprocal Packaging of the Main Structural Proteins of Type 1 Fimbriae and Flagella in the Outer Membrane Vesicles of "Wild Type" Escherichia coli Strains
- PMID: 33643229
- PMCID: PMC7907004
- DOI: 10.3389/fmicb.2021.557455
Reciprocal Packaging of the Main Structural Proteins of Type 1 Fimbriae and Flagella in the Outer Membrane Vesicles of "Wild Type" Escherichia coli Strains
Abstract
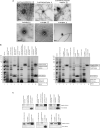
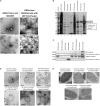
Fundamental aspects of outer membrane vesicle (OMV) biogenesis and the engineering of producer strains have been major research foci for many in recent years. The focus of this study was OMV production in a variety of Escherichia coli strains including wild type (WT) (K12 and BW25113), mutants (from the Keio collection) and proprietary [BL21 and BL21 (DE3)] strains. The present study investigated the proteome and prospective mechanism that underpinned the key finding that the dominant protein present in E. coli K-12 WT OMVs was fimbrial protein monomer (FimA) (a polymerizable protein which is the key structural monomer from which Type 1 fimbriae are made). However, mutations in genes involved in fimbriae biosynthesis (ΔfimA, B, C, and F) resulted in the packaging of flagella protein monomer (FliC) (the major structural protein of flagella) into OMVs instead of FimA. Other mutations (ΔfimE, G, H, I, and ΔlrhA-a transcriptional regulator of fimbriation and flagella biosynthesis) lead to the packaging of both FimA and Flagellin into the OMVs. In the majority of instances shown within this research, the production of OMVs is considered in K-12 WT strains where structural appendages including fimbriae or flagella are temporally co-expressed throughout the growth curve as shown previously in the literature. The hypothesis, proposed and supported within the present paper, is that the vesicular packaging of the major FimA is reciprocally regulated with the major FliC in E. coli K-12 OMVs but this is abrogated in a range of mutated, non-WT E. coli strains. We also demonstrate, that a protein of interest (GFP) can be targeted to OMVs in an E. coli K-12 strain by protein fusion with FimA and that this causes normal packaging to be disrupted. The findings and underlying implications for host interactions and use in biotechnology are discussed.
Keywords: Escherichia coli; FimA; Flagellin; FliC; OMV.
Copyright © 2021 Blackburn, Shepherd and Robinson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Flagella proteins contribute to the production of outer membrane vesicles from Escherichia coli W3110.Biochem Biophys Res Commun. 2013 Nov 8;441(1):151-6. doi: 10.1016/j.bbrc.2013.10.022. Epub 2013 Oct 14. Biochem Biophys Res Commun. 2013. PMID: 24134841
-
Quantitative Evaluation of Recombinant Protein Packaged into Outer Membrane Vesicles of Escherichia coli Cells.Biotechnol Prog. 2018 Jan;34(1):51-57. doi: 10.1002/btpr.2536. Epub 2017 Aug 21. Biotechnol Prog. 2018. PMID: 28786214
-
Comparative Analysis of Outer Membrane Vesicle Isolation Methods With an Escherichia coli tolA Mutant Reveals a Hypervesiculating Phenotype With Outer-Inner Membrane Vesicle Content.Front Microbiol. 2021 Mar 5;12:628801. doi: 10.3389/fmicb.2021.628801. eCollection 2021. Front Microbiol. 2021. PMID: 33746922 Free PMC article.
-
Construction of hypervesiculation Escherichia coli strains and application for secretory protein production.Biotechnol Bioeng. 2020 Mar;117(3):701-709. doi: 10.1002/bit.27239. Epub 2019 Dec 13. Biotechnol Bioeng. 2020. PMID: 31788781
-
Physical Extraction and Fast Protein Liquid Chromatography for Purifying Flagella Filament From Uropathogenic Escherichia coli for Immune Assay.Front Cell Infect Microbiol. 2019 Apr 24;9:118. doi: 10.3389/fcimb.2019.00118. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31069177 Free PMC article.
Cited by
-
Editorial: What is known and what remains to be discovered about bacterial outer membrane vesicles, volume II.Front Microbiol. 2022 Oct 3;13:929696. doi: 10.3389/fmicb.2022.929696. eCollection 2022. Front Microbiol. 2022. PMID: 36262321 Free PMC article. No abstract available.
-
High-yield vesicle-packaged recombinant protein production from E. coli.Cell Rep Methods. 2023 Feb 2;3(2):100396. doi: 10.1016/j.crmeth.2023.100396. eCollection 2023 Feb 27. Cell Rep Methods. 2023. PMID: 36936078 Free PMC article.
-
Bacterial Surface Appendages Modulate the Antimicrobial Activity Induced by Nanoflake Surfaces on Titanium.Small. 2024 Jun;20(26):e2310149. doi: 10.1002/smll.202310149. Epub 2024 Jan 17. Small. 2024. PMID: 38233200 Free PMC article.
-
Extraction of Bacterial Membrane Vesicle and Phage Complex by Density Gradient Ultracentrifugation.Bio Protoc. 2024 Aug 20;14(16):e5050. doi: 10.21769/BioProtoc.5050. eCollection 2024 Aug 20. Bio Protoc. 2024. PMID: 39210957 Free PMC article.
-
An Outer Membrane Vesicle-Based Permeation Assay (OMPA) for Assessing Bacterial Bioavailability.Adv Healthc Mater. 2022 Mar;11(5):e2101180. doi: 10.1002/adhm.202101180. Epub 2021 Oct 24. Adv Healthc Mater. 2022. PMID: 34614289 Free PMC article.
References
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., et al. (1994). Current Protocols in Molecular Biology. New York, NY: Greene Publishing Associates and Wiley-Interscience.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

