ORMDL2 Deficiency Potentiates the ORMDL3-Dependent Changes in Mast Cell Signaling
- PMID: 33643282
- PMCID: PMC7905224
- DOI: 10.3389/fimmu.2020.591975
ORMDL2 Deficiency Potentiates the ORMDL3-Dependent Changes in Mast Cell Signaling
Abstract
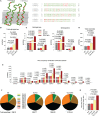
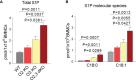
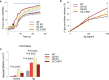
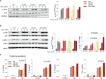
The systemic anaphylactic reaction is a life-threatening allergic response initiated by activated mast cells. Sphingolipids are an essential player in the development and attenuation of this response. De novo synthesis of sphingolipids in mammalian cells is inhibited by the family of three ORMDL proteins (ORMDL1, 2, and 3). However, the cell and tissue-specific functions of ORMDL proteins in mast cell signaling are poorly understood. This study aimed to determine cross-talk of ORMDL2 and ORMDL3 proteins in IgE-mediated responses. To this end, we prepared mice with whole-body knockout (KO) of Ormdl2 and/or Ormdl3 genes and studied their role in mast cell-dependent activation events in vitro and in vivo. We found that the absence of ORMDL3 in bone marrow-derived mast cells (BMMCs) increased the levels of cellular sphingolipids. Such an increase was further raised by simultaneous ORMDL2 deficiency, which alone had no effect on sphingolipid levels. Cells with double ORMDL2 and ORMDL3 KO exhibited increased intracellular levels of sphingosine-1-phosphate (S1P). Furthermore, we found that concurrent ORMDL2 and ORMDL3 deficiency increased IκB-α phosphorylation, degranulation, and production of IL-4, IL-6, and TNF-α cytokines in antigen-activated mast cells. Interestingly, the chemotaxis towards antigen was increased in all mutant cell types analyzed. Experiments in vivo showed that passive cutaneous anaphylaxis (PCA), which is initiated by mast cell activation, was increased only in ORMDL2,3 double KO mice, supporting our in vitro observations with mast cells. On the other hand, ORMDL3 KO and ORMDL2,3 double KO mice showed faster recovery from passive systemic anaphylaxis, which could be mediated by increased levels of blood S1P presented in such mice. Our findings demonstrate that Ormdl2 deficiency potentiates the ORMDL3-dependent changes in mast cell signaling.
Keywords: FcϵRI; ORMDL family; mast cells; passive cutaneous anaphylactic reaction; passive systemic anaphylaxis; sphingolipids; sphingosine-1-phosphate.
Copyright © 2021 Bugajev, Halova, Demkova, Cernohouzova, Vavrova, Mrkacek, Utekal, Draberova, Kuchar, Schuster and Draber.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Simultaneous reduction of all ORMDL proteins decreases the threshold of mast cell activation.Sci Rep. 2023 Jun 14;13(1):9615. doi: 10.1038/s41598-023-36344-5. Sci Rep. 2023. PMID: 37316542 Free PMC article.
-
Simultaneous deletion of ORMDL1 and ORMDL3 proteins disrupts immune cell homeostasis.Front Immunol. 2024 Apr 23;15:1376629. doi: 10.3389/fimmu.2024.1376629. eCollection 2024. Front Immunol. 2024. PMID: 38715613 Free PMC article.
-
Negative regulatory roles of ORMDL3 in the FcεRI-triggered expression of proinflammatory mediators and chemotactic response in murine mast cells.Cell Mol Life Sci. 2016 Mar;73(6):1265-85. doi: 10.1007/s00018-015-2047-3. Epub 2015 Sep 25. Cell Mol Life Sci. 2016. PMID: 26407610 Free PMC article.
-
Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model.Prostaglandins Other Lipid Mediat. 2008 Jun;86(1-4):1-11. doi: 10.1016/j.prostaglandins.2008.02.005. Epub 2008 Mar 4. Prostaglandins Other Lipid Mediat. 2008. PMID: 18403224 Free PMC article. Review.
-
The roles of sphingosine-1-phosphate in asthma.Mol Immunol. 2002 Sep;38(16-18):1239-45. doi: 10.1016/s0161-5890(02)00070-6. Mol Immunol. 2002. PMID: 12217390 Review.
Cited by
-
The Crosstalk between FcεRI and Sphingosine Signaling in Allergic Inflammation.Int J Mol Sci. 2022 Nov 11;23(22):13892. doi: 10.3390/ijms232213892. Int J Mol Sci. 2022. PMID: 36430378 Free PMC article. Review.
-
Simultaneous reduction of all ORMDL proteins decreases the threshold of mast cell activation.Sci Rep. 2023 Jun 14;13(1):9615. doi: 10.1038/s41598-023-36344-5. Sci Rep. 2023. PMID: 37316542 Free PMC article.
-
Neuroinflammation underlies the development of social stress induced cognitive deficit in male sickle cell mice.Exp Biol Med (Maywood). 2024 Nov 19;249:10361. doi: 10.3389/ebm.2024.10361. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39629138 Free PMC article.
-
RACK1 plays a critical role in mast cell secretion and Ca2+ mobilization by modulating F-actin dynamics.J Cell Sci. 2021 May 1;134(9):jcs252585. doi: 10.1242/jcs.252585. Epub 2021 May 13. J Cell Sci. 2021. PMID: 34550354 Free PMC article.
-
Simultaneous deletion of ORMDL1 and ORMDL3 proteins disrupts immune cell homeostasis.Front Immunol. 2024 Apr 23;15:1376629. doi: 10.3389/fimmu.2024.1376629. eCollection 2024. Front Immunol. 2024. PMID: 38715613 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

