Interleukin 17 Promotes Expression of Alarmins S100A8 and S100A9 During the Inflammatory Response of Keratinocytes
- PMID: 33643287
- PMCID: PMC7906991
- DOI: 10.3389/fimmu.2020.599947
Interleukin 17 Promotes Expression of Alarmins S100A8 and S100A9 During the Inflammatory Response of Keratinocytes
Abstract
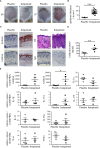
Psoriasis is one of the most common immune-mediated inflammatory skin diseases. Expression and secretion of two pro-inflammatory molecules of the S100-alarmin family, S100A8 and S100A9, in keratinocytes is a hallmark of psoriasis, which is also characterized by an altered differentiation of keratinocytes. Dimers of S100A8/S100A9 (calprotectin) bind to Toll-like receptor 4 and induce an inflammatory response in target cells. Targeted deletion of S100A9 reduced the inflammatory phenotype of psoriasis-like inflammation in mice. A role of S100-alarmins in differentiation and activation of keratinocytes was suggested but has been never shown in primary keratinocytes. We now confirm that induction of S100-alarmins in an imiquimod-induced murine model of psoriasis-like skin inflammation was associated with an increased expression of interleukin (IL)-1α, IL-6, IL-17A, or TNFα. This association was confirmed in transcriptome data obtained from controls, lesional and non-lesional skin of psoriasis patients, and a down-regulation of S100-alarmin expression after IL-17 directed therapy. However, analyzing primary S100A9-/- keratinocytes we found that expression of S100A8/S100A9 has no significant role for the maturation and inflammatory response pattern of keratinocytes. Moreover, keratinocytes are no target cells for the pro-inflammatory effects of S100A8/S100A9. However, different cytokines, especially IL-17A and F, highly abundant in psoriasis, strongly induced expression of S100-alarmins preferentially during early maturation stages of keratinocytes. Our data indicate that expression of S100A8 and S100A9 does not primarily influence maturation or activation of keratinocytes but rather represents the inflammatory response of these cells during psoriasis.
Keywords: S100A8; MRP14; MRP8; S100A9; calprotectin; keratinocytes; myeloid-related proteins; psoriasis.
Copyright © 2021 Christmann, Zenker, Martens, Hübner, Loser, Vogl and Roth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

