Deposition of the Membrane Attack Complex in Healthy and Diseased Human Kidneys
- PMID: 33643288
- PMCID: PMC7906018
- DOI: 10.3389/fimmu.2020.599974
Deposition of the Membrane Attack Complex in Healthy and Diseased Human Kidneys
Abstract
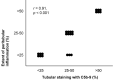
The membrane attack complex-also known as C5b-9-is the end-product of the classical, lectin, and alternative complement pathways. It is thought to play an important role in the pathogenesis of various kidney diseases by causing cellular injury and tissue inflammation, resulting in sclerosis and fibrosis. These deleterious effects are, consequently, targeted in the development of novel therapies that inhibit the formation of C5b-9, such as eculizumab. To clarify how C5b-9 contributes to kidney disease and to predict which patients benefit from such therapy, knowledge on deposition of C5b-9 in the kidney is essential. Because immunohistochemical staining of C5b-9 has not been routinely conducted and never been compared across studies, we provide a review of studies on deposition of C5b-9 in healthy and diseased human kidneys. We describe techniques to stain deposits and compare the occurrence of deposits in healthy kidneys and in a wide spectrum of kidney diseases, including hypertensive nephropathy, diabetic nephropathy, membranous nephropathy, IgA nephropathy, lupus nephritis, C3 glomerulopathy, and thrombotic microangiopathies such as the atypical hemolytic uremic syndrome, vasculitis, interstitial nephritis, acute tubular necrosis, kidney tumors, and rejection of kidney transplants. We summarize how these deposits are related with other histological lesions and clinical characteristics. We evaluate the prognostic relevance of these deposits in the light of possible treatment with complement inhibitors.
Keywords: C5b-9 (membrane attack complex [MAC]); biopsy; clinicopathological correlation; glomerular disease; histopathology; immunofluorescence; immunohistochemistry; renal.
Copyright © 2021 Koopman, van Essen, Rennke, de Vries and van Kooten.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Koopman JJE, Teng YKO, Boon CJF, Van den Heuvel LP, Rabelink TJ, Van Kooten C, et al. Diagnosis and treatment of C3 glomerulopathy in a center of expertise. Neth J Med (2019) 77:10–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

