The Role of Alpha 2 Macroglobulin in IgG-Aggregation and Chronic Activation of the Complement System in Patients With Chronic Lymphocytic Leukemia
- PMID: 33643290
- PMCID: PMC7905172
- DOI: 10.3389/fimmu.2020.603569
The Role of Alpha 2 Macroglobulin in IgG-Aggregation and Chronic Activation of the Complement System in Patients With Chronic Lymphocytic Leukemia
Abstract
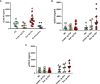
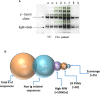
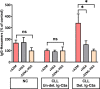
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the western world. One of the treatments offered for CLL is immunotherapy. These treatments activate various cellular and biochemical mechanisms, using the complement system. Recently it was shown that the complement system in CLL patients is persistently activated at a low level through the classical pathway (CP). The mechanism of chronic CP activation involves the formation of IgG-hexamers (IgG-aggregates). According to recent studies, formation of ordered IgG-hexamers occurs on cell surfaces via specific interactions between Fc regions of the IgG monomers, which occur after antigen binding. The present study investigated the formation of IgG-hexamers in CLL patients and normal (non-malignant) controls (NC), their ability to activate complement, their incidence as cell-free and cell-bound forms and the identity of the antigen causing their formation. Sera from 30 patients and 12 NC were used for separation of IgG- aggregates. The obtained IgG- aggregates were measured and used for assessment of CP activation. For evaluation of the presence of IgG- aggregates on blood cells, whole blood samples were stained and assessed by flow cytometry. Serum levels of IgG- aggregates were higher in CLL and they activated the complement system to a higher extent than in NC. Alpha 2 macroglobulin (A2M) was identified as the antigen causing the hexamerization/aggregation of IgG, and was found to be part of the hexamer structure by mass spectrometry, Western blot and flow cytometry analysis. The presence of A2M-IgG-hexamers on B-cells suggests that it may be formed on B cells surface and then be detached to become cell-free. Alternatively, it may form in the plasma and then attach to the cell surface. The exact time course of A2M-IgG-hexamers formation in CLL should be further studied. The results in this study may be useful for improvement of current immunotherapy regimens.
Keywords: IgG-hexamers,; alpha 2 macroglobulin; chronic lymphocytic leukemia; classical pathway; complement system.
Copyright © 2021 Naseraldeen, Michelis, Barhoum, Chezar, Tadmor, Aviv, Shvidel, Litmanovich, Shehadeh, Stemer, Shaoul and Braester.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Chien WW, Niogret C, Jugé R, Lionnard L, Cornut-Thibaut A, Kucharczak J, et al. Unexpected cross-reactivity of anti-cathepsin B antibodies leads to uncertainties regarding the mechanism of action of anti-CD20 monoclonal antibody GA101. Leuk Res (2017) 55:41–8. 10.1016/j.leukres.2017.01.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

